A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00685-0, NCT04950803, Dec 2023
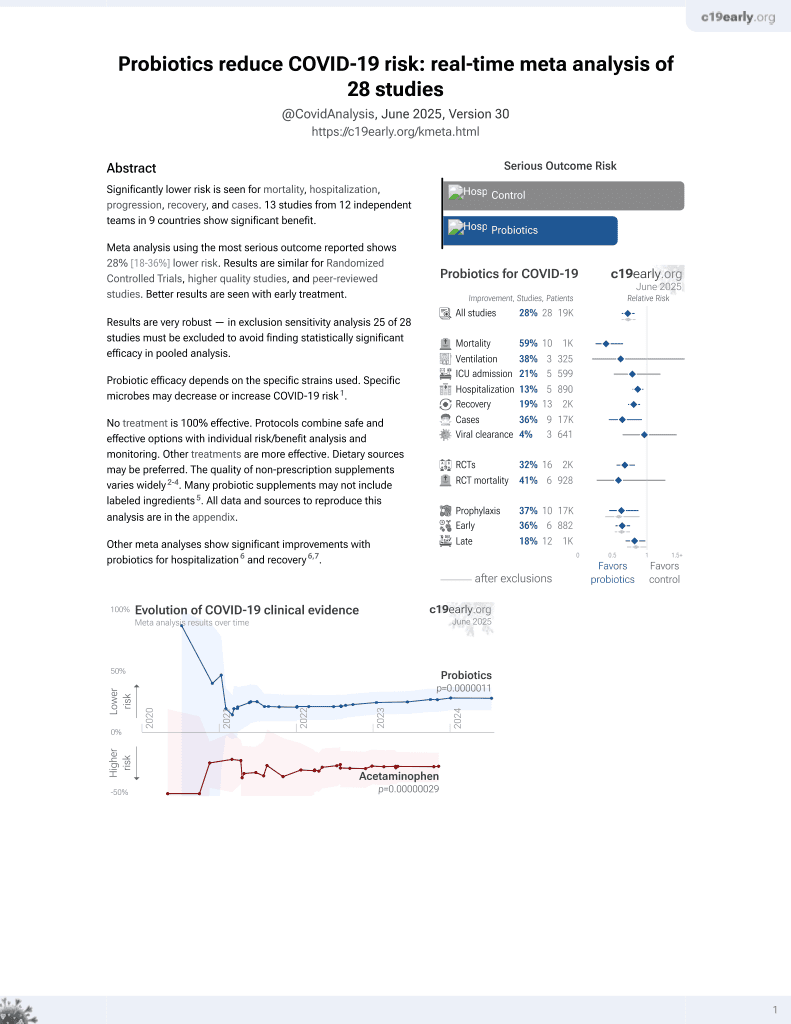
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
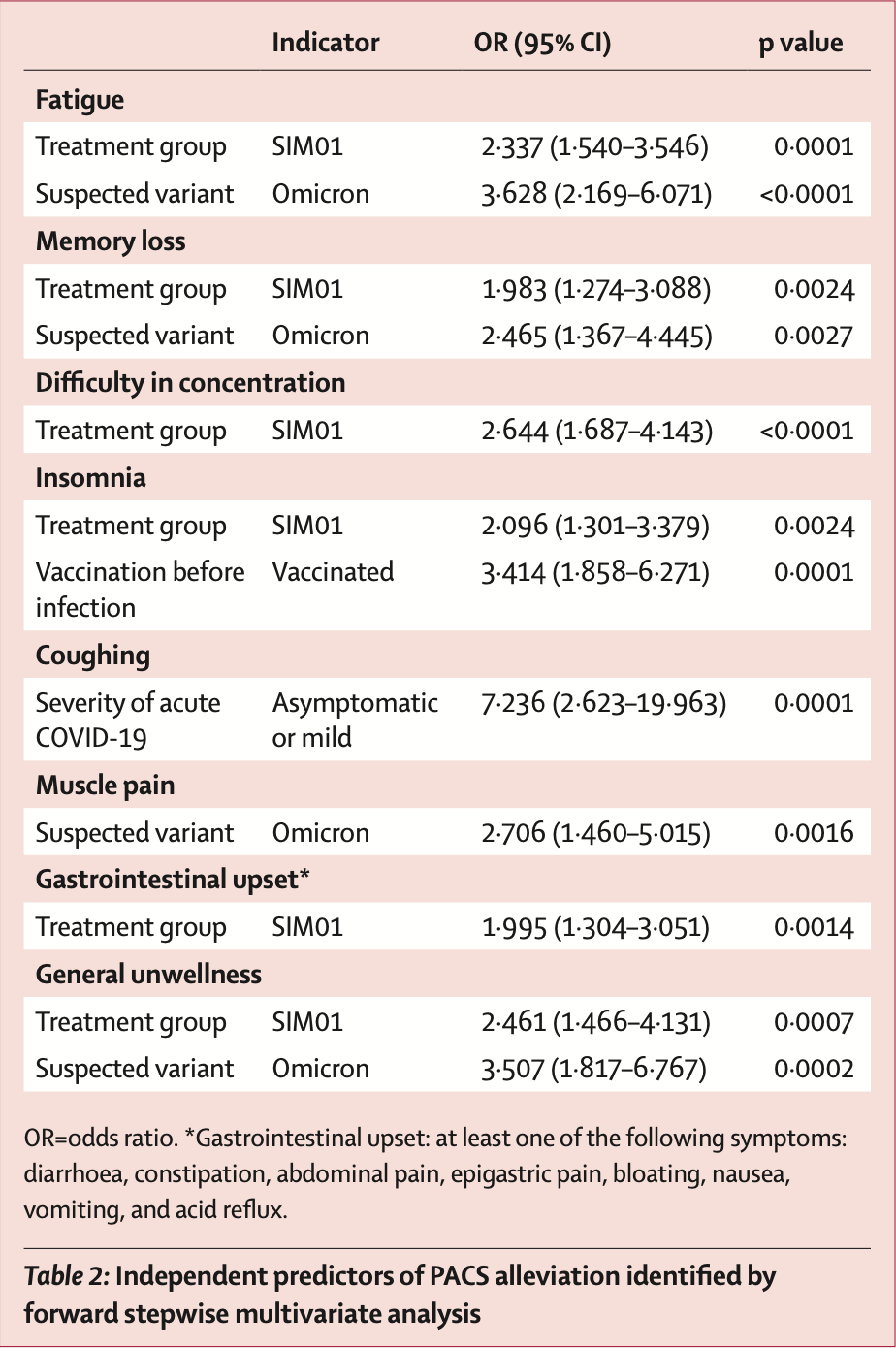
RCT 463 patients with post-acute COVID-19 syndrome (PACS) showing that treatment with a synbiotic preparation (SIM01) containing probiotics and prebiotics was associated with alleviation of multiple PACS symptoms including fatigue, memory loss, difficulty concentrating, gastrointestinal upset, and general unwellness compared to placebo.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments2.
|
risk of no recovery, 57.2% lower, OR 0.43, p < 0.001, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, fatigue, RR approximated with OR.
|
|
risk of no recovery, 49.6% lower, OR 0.50, p = 0.002, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, memory loss, RR approximated with OR.
|
|
risk of no recovery, 62.2% lower, OR 0.38, p < 0.001, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, concentration, RR approximated with OR.
|
|
risk of no recovery, 52.3% lower, OR 0.48, p = 0.002, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, insomnia, RR approximated with OR.
|
|
risk of no recovery, 49.9% lower, OR 0.50, p = 0.001, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, gastrointestinal upset, RR approximated with OR.
|
|
risk of no recovery, 59.4% lower, OR 0.41, p < 0.001, treatment 232, control 231, adjusted per study, inverted to make OR<1 favor treatment, multivariable, general unwellness, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lau et al., 7 Dec 2023, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, 16 authors, study period 25 June, 2021 - 12 August, 2022, trial NCT04950803 (history).
Contact: siewchienng@cuhk.edu.hk.
A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(23)00685-0
Background Post-acute COVID-19 syndrome (PACS) affects over 65 million individuals worldwide but treatment options are scarce. We aimed to assess a synbiotic preparation (SIM01) for the alleviation of PACS symptoms.
Methods In this randomised, double-blind, placebo-controlled trial at a tertiary referral centre in Hong Kong, patients with PACS according to the US Centers for Disease Control and Prevention criteria were randomly assigned (1:1) by random permuted blocks to receive SIM01 (10 billion colony-forming units in sachets twice daily) or placebo orally for 6 months. Inclusion criterion was the presence of at least one of 14 PACS symptoms for 4 weeks or more after confirmed SARS-CoV-2 infection, including fatigue, memory loss, difficulty in concentration, insomnia, mood disturbance, hair loss, shortness of breath, coughing, inability to exercise, chest pain, muscle pain, joint pain, gastrointestinal upset, or general unwellness. Individuals were excluded if they were immunocompromised, were pregnant or breastfeeding, were unable to receive oral fluids, or if they had received gastrointestinal surgery in the 30 days before randomisation. Participants, care providers, and investigators were masked to group assignment. The primary outcome was alleviation of PACS symptoms by 6 months, assessed by an interviewer-administered 14-item questionnaire in the intention-to-treat population. Forward stepwise multivariable logistical regression was performed to identify predictors of symptom alleviation. The trial is registered with ClinicalTrials.gov, NCT04950803.
Findings Between June 25, 2021, and Aug 12, 2022, 463 patients were randomly assigned to receive SIM01 (n=232) or placebo (n=231). At 6 months, significantly higher proportions of the SIM01 group had alleviation of fatigue (OR 2•273, 95% CI 1•520-3•397, p=0•0001), memory loss (1•967, 1•271-3•044, p=0•0024), difficulty in concentration (2•644, 1•687-4•143, p<0•0001), gastrointestinal upset (1•995, 1•304-3•051, p=0•0014), and general unwellness (2•360, 1•428-3•900, p=0•0008) compared with the placebo group. Adverse event rates were similar between groups during treatment (SIM01 22 [10%] of 232 vs placebo 25 [11%] of 231; p=0•63). Treatment with SIM01, infection with omicron variants, vaccination before COVID-19, and mild acute COVID-19, were predictors of symptom alleviation (p<0•0036). Interpretation Treatment with SIM01 alleviates multiple symptoms of PACS. Our findings have implications on the management of PACS through gut microbiome modulation. Further studies are warranted to explore the beneficial effects of SIM01 in other chronic or post-infection conditions.
Contributors RIL and QS conceived the study, accessed and verified all the data, and prepared the manuscript. ISFL, MCSW, LHSL, and SWHC contributed to participant recruitment and clinical assessment. JYLC contributed to clinical data management and study monitoring. HMT and CKPM contributed to cytokines profiling. YKT provided important comments on statistical analysis. CPC contributed to sample collection and biobank management. MKTL contributed to sample processing and metagenomic sequencing. GTYY and PKC provided important assistance during the clinical trial. FKLC and SCN designed the study and contributed to data interpretation and manuscript writing. All authors read, revised, and had final responsibility for the decision to submit the manuscript for publication and had full access to all the data in the study.
Declaration of interests All authors have completed the Unified Competing Interest form (available on request to the corresponding author). MCSW is an advisory committee member of Pfizer, an external expert of GlaxoSmithKline, a member of the advisory board of AstraZeneca, and has been paid consultancy fees for providing advice on research. LHSL is supported by grants from the Health and Medical Research Fund, General Research Fund, and Direct Grant for Research; received honoraria as a speaker for Olympus, Boston Scientific, and GenieBiome; travel grants from Olympus, Pfizer, and AbbVie; and received research product support from GenieBiome for his other..
References
Austhof, Bell, Riddle, Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: results from the Arizona CoVHORT, Epidemiol Infect
Craig, Marshall, Sjöström, International physical activity questionnaire: 12-country reliability and validity, Med Sci Sports Exerc
Crook, Raza, Nowell, Young, Long covidmechanisms, risk factors, and management, BMJ
Cryan, 'riordan, Cowan, The microbiota-gutbrain axis, Physiol Rev
Dale, Rasmussen, Asiller, Lied, Probiotics in irritable bowel syndrome: an up-to-date systematic review, Nutrients
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
Lee, Annamalai, Rao, Post-infectious irritable bowel syndrome, Curr Gastroenterol Rep
Lew, Hor, Yusoff, Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebocontrolled study, Clin Nutr
Li, Summanen, Komoriya, Finegold, In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp, Int J Food Sci Nutr
Liu, Mak, Su, Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome, Gut
Marasco, Cremon, Barbaro, Post COVID-19 irritable bowel syndrome, Gut
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med
Nashed, Mani, Hazrati, Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection, Gut
Sarkar, Mazumder, Banerjee, Probiotics protect against gut dysbiosis associated decline in learning and memory, J Neuroimmunol
Sencio, Machelart, Robil, Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters, Gut Microbes
Sokol, Contreras, Maisonnasse, SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota, Gut Microbes
Su, Lau, Liu, Chan, Ng, Post-acute COVID-19 syndrome and gut dysbiosis linger beyond 1 year after SARS-CoV-2 clearance, Gut
Sukocheva, Maksoud, Beeraka, Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome, J Adv Res
Wilson, Rossi, Dimidi, Whelan, Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials, Am J Clin Nutr
Wong, Zhang, Ching, Effects of gut microbiome modulation on reducing adverse health outcomes among elderly and diabetes patients during the COVID-19 pandemic: a randomised, double-blind, placebo-controlled trial (IMPACT Study), Nutrients
Xie, Edwards, Adam, Resurgence of omicron BA.2 in SARS-CoV-2 infection-naive Hong Kong, Nat Commun
Xiong, Gunter, Fleming, Multi-'omics of gut microbiome-host interactions in short-and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients, Cell Host Microbe
Yang, Yu, Xue, Li, Du, Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice, Acta Pharm Sin B
Yeoh, Zuo, Lui, Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1016/S1473-3099(23)00685-0
Zhang, Lau, Liu, Su, Chan et al., Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications, Nat Rev Gastroenterol Hepatol
Zhang, Wan, Zuo, Prolonged impairment of short-chain fatty acid and L-isoleucine biosynthesis in gut microbiome in patients with COVID-19, Gastroenterology
Zhang, Xu, Mak, Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: an open-label pilot study, J Gastroenterol Hepatol
Zuo, Zhang, Lui, Alterations in gut microbiota of patients with COVID-19 during time of hospitalization, Gastroenterology
Śliżewska, Libudzisz, Barczyńska, Kapuśniak, Zduńczyk et al., Dietary resistant dextrins positively modulate fecal and cecal microbiota composition in young rats, Acta Biochim Pol
DOI record:
{
"DOI": "10.1016/s1473-3099(23)00685-0",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(23)00685-0",
"alternative-id": [
"S1473309923006850"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(23)00685-0"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(23)00735-1"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Lau",
"given": "Raphaela I",
"sequence": "first"
},
{
"affiliation": [],
"family": "Su",
"given": "Qi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Ivan S F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ching",
"given": "Jessica Y L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Martin C S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Louis H S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tun",
"given": "Hein M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mok",
"given": "Chris K P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chau",
"given": "Steven W H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tse",
"given": "Yee Kit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheung",
"given": "Chun Pan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Moses K T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yeung",
"given": "Giann T Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheong",
"given": "Pui Kuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Francis K L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ng",
"given": "Siew C",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
7
]
],
"date-time": "2023-12-07T23:33:11Z",
"timestamp": 1701991991000
},
"deposited": {
"date-parts": [
[
2023,
12,
7
]
],
"date-time": "2023-12-07T23:33:25Z",
"timestamp": 1701992005000
},
"funder": [
{
"DOI": "10.13039/501100016080",
"doi-asserted-by": "publisher",
"name": "Hui Hoy and Chow Sin Lan Charity Fund Limited"
},
{
"DOI": "10.13039/501100017649",
"doi-asserted-by": "publisher",
"name": "Government of the Hong Kong Special Administrative Region of the People"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
8
]
],
"date-time": "2023-12-08T00:46:16Z",
"timestamp": 1701996376685
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923006850?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923006850?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations",
"author": "Davis",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/S1473-3099(23)00685-0_bib1",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"article-title": "Post-acute COVID-19 syndrome",
"author": "Nalbandian",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "Nat Med",
"key": "10.1016/S1473-3099(23)00685-0_bib3",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41575-022-00698-4",
"article-title": "Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Nat Rev Gastroenterol Hepatol",
"key": "10.1016/S1473-3099(23)00685-0_bib4",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1136/gutjnl-2021-325989",
"article-title": "Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Gut",
"key": "10.1016/S1473-3099(23)00685-0_bib5",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2022-328319",
"article-title": "Post-acute COVID-19 syndrome and gut dysbiosis linger beyond 1 year after SARS-CoV-2 clearance",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "1230",
"journal-title": "Gut",
"key": "10.1016/S1473-3099(23)00685-0_bib6",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1111/jgh.15796",
"article-title": "Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: an open-label pilot study",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "823",
"journal-title": "J Gastroenterol Hepatol",
"key": "10.1016/S1473-3099(23)00685-0_bib7",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.3390/nu15081982",
"article-title": "Effects of gut microbiome modulation on reducing adverse health outcomes among elderly and diabetes patients during the COVID-19 pandemic: a randomised, double-blind, placebo-controlled trial (IMPACT Study)",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "Nutrients",
"key": "10.1016/S1473-3099(23)00685-0_bib8",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "10.1016/S1473-3099(23)00685-0_bib9",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2021.10.013",
"article-title": "Prolonged impairment of short-chain fatty acid and L-isoleucine biosynthesis in gut microbiome in patients with COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "548",
"journal-title": "Gastroenterology",
"key": "10.1016/S1473-3099(23)00685-0_bib10",
"volume": "162",
"year": "2022"
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"article-title": "Alterations in gut microbiota of patients with COVID-19 during time of hospitalization",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "944",
"journal-title": "Gastroenterology",
"key": "10.1016/S1473-3099(23)00685-0_bib11",
"volume": "159",
"year": "2020"
},
{
"article-title": "Resurgence of omicron BA.2 in SARS-CoV-2 infection-naive Hong Kong",
"author": "Xie",
"journal-title": "Nat Commun",
"key": "10.1016/S1473-3099(23)00685-0_bib12",
"volume": "14",
"year": "2023"
},
{
"article-title": "Long covid-mechanisms, risk factors, and management",
"author": "Crook",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(23)00685-0_bib13",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1249/01.MSS.0000078924.61453.FB",
"article-title": "International physical activity questionnaire: 12-country reliability and validity",
"author": "Craig",
"doi-asserted-by": "crossref",
"first-page": "1381",
"journal-title": "Med Sci Sports Exerc",
"key": "10.1016/S1473-3099(23)00685-0_bib14",
"volume": "35",
"year": "2003"
},
{
"DOI": "10.1007/s11894-017-0595-4",
"article-title": "Post-infectious irritable bowel syndrome",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Curr Gastroenterol Rep",
"key": "10.1016/S1473-3099(23)00685-0_bib15",
"volume": "19",
"year": "2017"
},
{
"article-title": "Post COVID-19 irritable bowel syndrome",
"author": "Marasco",
"journal-title": "Gut",
"key": "10.1016/S1473-3099(23)00685-0_bib16",
"year": "2022"
},
{
"DOI": "10.1017/S0950268822001200",
"article-title": "Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: results from the Arizona CoVHORT",
"author": "Austhof",
"doi-asserted-by": "crossref",
"first-page": "e136",
"journal-title": "Epidemiol Infect",
"key": "10.1016/S1473-3099(23)00685-0_bib17",
"volume": "150",
"year": "2022"
},
{
"DOI": "10.3390/nu11092048",
"article-title": "Probiotics in irritable bowel syndrome: an up-to-date systematic review",
"author": "Dale",
"doi-asserted-by": "crossref",
"journal-title": "Nutrients",
"key": "10.1016/S1473-3099(23)00685-0_bib18",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/j.jare.2021.11.013",
"article-title": "Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome",
"author": "Sukocheva",
"doi-asserted-by": "crossref",
"first-page": "179",
"journal-title": "J Adv Res",
"key": "10.1016/S1473-3099(23)00685-0_bib19",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2023.01.001",
"article-title": "Multi-'omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients",
"author": "Xiong",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Cell Host Microbe",
"key": "10.1016/S1473-3099(23)00685-0_bib20",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1152/physrev.00018.2018",
"article-title": "The microbiota–gut–brain axis",
"author": "Cryan",
"doi-asserted-by": "crossref",
"first-page": "1877",
"journal-title": "Physiol Rev",
"key": "10.1016/S1473-3099(23)00685-0_bib21",
"volume": "99",
"year": "2019"
},
{
"DOI": "10.1016/j.clnu.2018.09.010",
"article-title": "Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study",
"author": "Lew",
"doi-asserted-by": "crossref",
"first-page": "2053",
"journal-title": "Clin Nutr",
"key": "10.1016/S1473-3099(23)00685-0_bib22",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.1016/j.jneuroim.2020.577390",
"article-title": "Probiotics protect against gut dysbiosis associated decline in learning and memory",
"author": "Roy Sarkar",
"doi-asserted-by": "crossref",
"journal-title": "J Neuroimmunol",
"key": "10.1016/S1473-3099(23)00685-0_bib23",
"volume": "348",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2019.07.001",
"article-title": "Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "475",
"journal-title": "Acta Pharm Sin B",
"key": "10.1016/S1473-3099(23)00685-0_bib24",
"volume": "10",
"year": "2020"
},
{
"article-title": "Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters",
"author": "Sencio",
"journal-title": "Gut Microbes",
"key": "10.1016/S1473-3099(23)00685-0_bib25",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1080/19490976.2021.1893113",
"article-title": "SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota",
"author": "Sokol",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Gut Microbes",
"key": "10.1016/S1473-3099(23)00685-0_bib26",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2021-326599",
"article-title": "Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection",
"author": "Nashed",
"doi-asserted-by": "crossref",
"first-page": "2371",
"journal-title": "Gut",
"key": "10.1016/S1473-3099(23)00685-0_bib27",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.3109/09637486.2015.1064869",
"article-title": "In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "919",
"journal-title": "Int J Food Sci Nutr",
"key": "10.1016/S1473-3099(23)00685-0_bib28",
"volume": "66",
"year": "2015"
},
{
"DOI": "10.18388/abp.2015_1101",
"article-title": "Dietary resistant dextrins positively modulate fecal and cecal microbiota composition in young rats",
"author": "Śliżewska",
"doi-asserted-by": "crossref",
"first-page": "677",
"journal-title": "Acta Biochim Pol",
"key": "10.1016/S1473-3099(23)00685-0_bib29",
"volume": "62",
"year": "2015"
},
{
"DOI": "10.1093/ajcn/nqy376",
"article-title": "Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials",
"author": "Wilson",
"doi-asserted-by": "crossref",
"first-page": "1098",
"journal-title": "Am J Clin Nutr",
"key": "10.1016/S1473-3099(23)00685-0_bib30",
"volume": "109",
"year": "2019"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309923006850"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}