Association of nirmatrelvir for acute SARS‐CoV‐2 infection with subsequent Long COVID symptoms in an observational cohort study
et al., Journal of Medical Virology, doi:10.1002/jmv.29333, Jan 2024
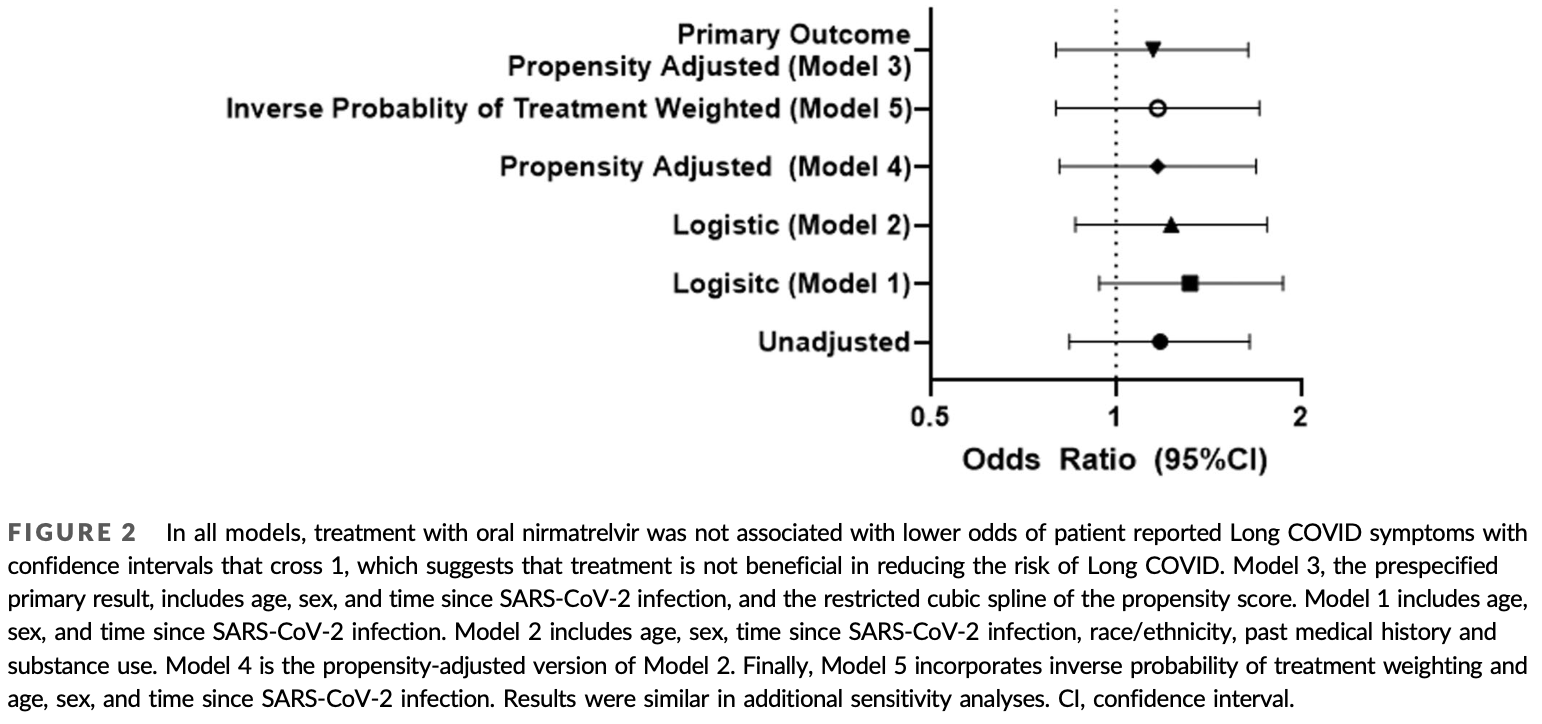
Retrospective 4,684 COVID+ patients mostly in the USA, 988 treated with paxlovid, showing higher risk of long COVID with treatment, without statistical significance.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 14.3% higher, RR 1.14, p = 0.40, treatment 57 of 353 (16.1%), control 176 of 1,258 (14.0%), odds ratio converted to relative risk, propensity score weighting, model 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Durstenfeld et al., 4 Jan 2024, retrospective, USA, peer-reviewed, 13 authors.
Contact: matthew.durstenfeld@ucsf.edu, covid19@eurekaplatform.org.
Association of nirmatrelvir for acute SARS‐CoV‐2 infection with subsequent Long COVID symptoms in an observational cohort study
Journal of Medical Virology, doi:10.1002/jmv.29333
Oral nirmatrelvir/ritonavir is approved as treatment for acute COVID-19, but the effect of treatment during acute infection on risk of Long COVID is unknown. We hypothesized that nirmatrelvir treatment during acute SARS-CoV-2 infection reduces risk of developing Long COVID and rebound after treatment is associated with Long COVID. We conducted an observational cohort study within the Covid Citizen Science (CCS) study, an online cohort study with over 100 000 participants. We included vaccinated, nonhospitalized, nonpregnant individuals who reported their first SARS-CoV-2 positive test March-August 2022. Oral nirmatrelvir/ritonavir treatment was ascertained during acute SARS-CoV-2 infection. Patient-reported Long COVID symptoms, symptom rebound and test-positivity rebound were asked on subsequent surveys at least 3 months after SARS-CoV-2 infection. A total of 4684 individuals met the eligibility criteria, of whom 988 (21.1%) were treated and 3696 (78.9%) were untreated; 353/988 (35.7%) treated and 1258/3696 (34.0%) untreated responded to the Long COVID survey (n = 1611). Among 1611 participants, median
The other authors report no conflicts of interest.
DATA AVAILABILITY STATEMENT The data that support the findings of this study are available from the corresponding author upon reasonable request. Data are available by application to the COVID Citizen Science leadership committee. Data may be requested by emailing covid19@eurekaplatform.org.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Antar, Yu, Demko, Long COVID brain fog and muscle pain are associated with longer time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute infection, Front Immunol, doi:10.3389/fimmu.2023.1147549
Apple, Oddi, Peluso, Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19, Ann Clin Transl Neurol
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and sixmonth outcomes, Ann Intern Med
Beatty, Peyser, Butcher, The COVID-19 citizen science study: protocol for a longitudinal digital health cohort study, JMIR Res Protoc
Congdon, Narrowe, Yone, Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study, Sci Rep
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a population-based cohort study, Ann Intern Med
Durstenfeld, Peluso, Kaveti, Reduced Exercise Capacity, Chronotropic incompetence, and early systemic inflammation in cardiopulmonary phenotype long coronavirus disease 2019, J Infect Dis, doi:10.1093/infdis/jiad131
Durstenfeld, Peluso, Peyser, Factors associated with long COVID symptoms in an online cohort study, Open Forum Infect Dis
Edelstein, Boucau, Uddin, SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy: an observational study, Ann Intern Med, doi:10.7326/M23-1756
Geng, Bonilla, Shafer, Miglis, Yang, The use of nirmatrelvir-ritonavir in a case of breakthrough long COVID, Explor Res Hypothesis Med, doi:10.14218/ERHM.2022.00045
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Hansen, Makkar, Sahner, Paxlovid (nirmatrelvir/ ritonavir) effectiveness against hospitalization and death in N3C: a target trial emulation study, medRxiv, doi:10.1101/2023.05.26.23290602
Hernán, Robins, Using big data to emulate a target trial when a randomized trial is not available, Am J Epidemiol
Najjar-Debbiny, Gronich, Weber, Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis
Natarajan, Zlitni, Brooks, Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection, Med
Patel, Dani, Khadke, Incidence of symptoms associated with post-acute sequelae of SARS-CoV-2 infection in nonhospitalized vaccinated patients receiving Nirmatrelvir-Ritonavir, medRxiv, doi:10.1101/2023.04.05.23288196
Peluso, Anglin, Durstenfeld, Effect of oral nirmatrelvir on Long COVID symptoms: 4 cases and rationale for systematic studies, Pathogens and Immunity
Peluso, Deeks, Mustapic, SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19, Ann Neurol
Peluso, Ryder, Flavell, Multimodal molecular imaging reveals tissue-based T cell activation and viral RNA persistence for up to 2 years following COVID-19, medRxiv, doi:10.1101/2023.07.27.23293177
Peluso, Swank, Goldberg, Plasma-based antigen persistence in the post-acute phase of SARS-CoV-2 infection. medRxiv, doi:10.1101/2023.10.24.23297114
Qi, Wang, Nirmatrelvir-ritonavir therapy and COVID-19 vaccination improve clinical outcomes of SARS-CoV-2 omicron variant infection, J Med Virol, doi:10.1002/jmv.28497
Ranganath, Horo, Challener, Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID-19) in high-risk persons, Clin Infect Dis
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19 -United States, April-September 2022, Am J Transplant (AJT)
Soriano, Murthy, Marshall, Relan, Diaz, A clinical case definition of post-COVID-19 condition by a Delphi consensus, The Lancet Infectious Diseases
Stein, Ramelli, Grazioli, SARS-CoV-2 infection and persistence in the human body and brain at autopsy, Nature
Su, Yuan, Chen, Multiple early factors anticipate postacute COVID-19 sequelae, Cell
Swank, Senussi, Manickas-Hill, Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae, Clin Infect Dis, doi:10.1093/cid/ciac722
Uhm, Ahn, Hyun, Patterns of viral clearance in the natural course of asymptomatic COVID-19: comparison with symptomatic non-severe COVID-19, Int J Infect Dis
Visvabharathy, Orban, Koralnik, Case report: treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence, Front Med
Von Elm, Altman, Egger, Pocock, Gøtzsche et al., The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Ann Intern Med
Wai, Chan, Cheung, Association of molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health West Pac
Wang, Zhao, Liu, Chen, Feng, Early administration of paxlovid reduces the viral elimination time in patients infected with SARS-CoV-2 omicron variants, J Med Virol
Watanabe, Iwagami, Yasuhara, Takagi, Kuno, Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis, Vaccine
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wong, Yip, Lai, Wong, Hui et al., Incidence of viral rebound after treatment with nirmatrelvir-ritonavir and molnupiravir, JAMA Network Open
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Int Med
DOI record:
{
"DOI": "10.1002/jmv.29333",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.29333",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Oral nirmatrelvir/ritonavir is approved as treatment for acute COVID‐19, but the effect of treatment during acute infection on risk of Long COVID is unknown. We hypothesized that nirmatrelvir treatment during acute SARS‐CoV‐2 infection reduces risk of developing Long COVID and rebound after treatment is associated with Long COVID. We conducted an observational cohort study within the Covid Citizen Science (CCS) study, an online cohort study with over 100 000 participants. We included vaccinated, nonhospitalized, nonpregnant individuals who reported their first SARS‐CoV‐2 positive test March–August 2022. Oral nirmatrelvir/ritonavir treatment was ascertained during acute SARS‐CoV‐2 infection. Patient‐reported Long COVID symptoms, symptom rebound and test‐positivity rebound were asked on subsequent surveys at least 3 months after SARS‐CoV‐2 infection. A total of 4684 individuals met the eligibility criteria, of whom 988 (21.1%) were treated and 3696 (78.9%) were untreated; 353/988 (35.7%) treated and 1258/3696 (34.0%) untreated responded to the Long COVID survey (<jats:italic>n</jats:italic> = 1611). Among 1611 participants, median age was 55 years and 66% were female. At 5.4 ± 1.3 months after infection, nirmatrelvir treatment was not associated with subsequent Long COVID symptoms (odds ratio [OR]: 1.15; 95% confidence interval [CI]: 0.80–1.64; <jats:italic>p</jats:italic> = 0.45). Among 666 treated who answered rebound questions, rebound symptoms or test positivity were not associated with Long COVID symptoms (OR: 1.34; 95% CI: 0.74–2.41; <jats:italic>p</jats:italic> = 0.33). Within this cohort of vaccinated, nonhospitalized individuals, oral nirmatrelvir treatment during acute SARS‐CoV‐2 infection and rebound after nirmatrelvir treatment were not associated with Long COVID symptoms more than 90 days after infection.</jats:p>",
"alternative-id": [
"10.1002/jmv.29333"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-11-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-12-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-01-04"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7612-3352",
"affiliation": [
{
"name": "Division of Cardiology at ZSFG, Department of Medicine University of California, San Francisco (UCSF) San Francisco California USA"
}
],
"authenticated-orcid": false,
"family": "Durstenfeld",
"given": "Matthew S.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of HIV, Infectious Disease, & Global Medicine UCSF San Francisco California USA"
}
],
"family": "Peluso",
"given": "Michael J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology and Biostatistics UCSF San Francisco California USA"
}
],
"family": "Lin",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology and Biostatistics UCSF San Francisco California USA"
}
],
"family": "Peyser",
"given": "Noah D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology & Population Health Albert Einstein College of Medicine New York City New York USA"
}
],
"family": "Isasi",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Louisiana Public Health Institute New Orleans Louisiana USA"
}
],
"family": "Carton",
"given": "Thomas W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Experimental Medicine UCSF San Francisco California USA"
}
],
"family": "Henrich",
"given": "Timothy J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of HIV, Infectious Disease, & Global Medicine UCSF San Francisco California USA"
}
],
"family": "Deeks",
"given": "Steven G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Medicine UCSF San Francisco California USA"
}
],
"family": "Olgin",
"given": "Jeffrey E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology and Biostatistics UCSF San Francisco California USA"
}
],
"family": "Pletcher",
"given": "Mark J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Epidemiology and Biostatistics, Department of Medicine UCSF San Francisco California USA"
}
],
"family": "Beatty",
"given": "Alexis L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Medicine UCSF San Francisco California USA"
}
],
"family": "Marcus",
"given": "Gregory M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology at ZSFG, Department of Medicine University of California, San Francisco (UCSF) San Francisco California USA"
}
],
"family": "Hsue",
"given": "Priscilla Y.",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T15:00:36Z",
"timestamp": 1704380436000
},
"deposited": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T15:00:43Z",
"timestamp": 1704380443000
},
"funder": [
{
"DOI": "10.13039/100000070",
"doi-asserted-by": "publisher",
"name": "National Institute of Biomedical Imaging and Bioengineering"
},
{
"DOI": "10.13039/100000865",
"doi-asserted-by": "publisher",
"name": "Bill and Melinda Gates Foundation"
},
{
"DOI": "10.13039/100006093",
"doi-asserted-by": "publisher",
"name": "Patient-Centered Outcomes Research Institute"
},
{
"DOI": "10.13039/100000050",
"doi-asserted-by": "publisher",
"name": "National Heart, Lung, and Blood Institute"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
5
]
],
"date-time": "2024-01-05T00:15:25Z",
"timestamp": 1704413725754
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 3,
"start": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T00:00:00Z",
"timestamp": 1704326400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.29333",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
4
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S1473-3099(21)00703-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1016/j.vaccine.2023.02.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1016/j.cell.2022.01.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.3389/fimmu.2023.1147549",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1038/s41586-022-05542-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1093/cid/ciac722",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1016/j.medj.2022.04.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1002/ana.26350",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1101/2023.10.24.23297114",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_10_1",
"unstructured": "PelusoMJ SwankZN GoldbergSA et al.Plasma‐based antigen persistence in the post‐acute phase of SARS‐CoV‐2 infection.medRxiv. Preprint posted online October 26 2023.doi:10.1101/2023.10.24.23297114"
},
{
"DOI": "10.1101/2023.07.27.23293177",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_11_1",
"unstructured": "PelusoMJ RyderD FlavellR et al.Multimodal molecular imaging reveals tissue‐based T cell activation and viral RNA persistence for up to 2 years following COVID‐19.medRxiv. Preprint posted online July 31 2023.doi:10.1101/2023.07.27.23293177"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.20411/pai.v7i1.518",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.3389/fmed.2022.1003103",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.14218/ERHM.2022.00045",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_15_1",
"unstructured": "GengLN BonillaHF ShaferRW MiglisMG YangPC.The use of nirmatrelvir‐ritonavir in a case of breakthrough long COVID.Explor Res Hypothesis Med. Published online January 13 2023.doi:10.14218/ERHM.2022.00045"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.7326/M22-3565",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1038/s41598-023-46912-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.2196/28169",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1093/ofid/ofad047",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1093/aje/kwv254",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.7326/0003-4819-147-8-200710160-00010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"key": "e_1_2_10_23_1",
"unstructured": "Administration FaD. Fact Sheet for Healthcare Providers In:2022."
},
{
"key": "e_1_2_10_24_1",
"unstructured": "Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S. FDA [press release].2022."
},
{
"DOI": "10.1093/cid/ciac443",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.7326/M22-2141",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1016/j.ajt.2022.12.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1101/2023.05.26.23290602",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_30_1",
"unstructured": "HansenK MakkarSR SahnerD et al.Paxlovid (nirmatrelvir/ritonavir) effectiveness against hospitalization and death in N3C: a target trial emulation study.medRxiv. Preprint posted online June 3 2023.doi:10.1101/2023.05.26.23290602"
},
{
"DOI": "10.1002/jmv.28497",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1002/jmv.28443",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.2139/ssrn.4415655",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_33_1",
"unstructured": "PatelR DaniS KhadkeS et al.Incidence of symptoms associated with post‐acute sequelae of SARS‐CoV‐2 infection in non‐hospitalized vaccinated patients receiving Nirmatrelvir‐Ritonavir.medRxiv. Preprint posted online April 6 2023.doi:10.1101/2023.04.05.23288196"
},
{
"DOI": "10.1001/jamanetworkopen.2022.45086",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.1093/cid/ciac481",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"DOI": "10.7326/M23-1756",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_1"
},
{
"DOI": "10.1016/j.ijid.2020.07.070",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1093/infdis/jiad131",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1002/acn3.51498",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.29333"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Association of nirmatrelvir for acute SARS‐CoV‐2 infection with subsequent Long COVID symptoms in an observational cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "96"
}