Impact of Anti-angiogenic Drugs on Severity of COVID-19 in Patients with Non-Small Cell Lung Cancer
et al., Technology in Cancer Research & Treatment, doi:10.1177/15330338241248573, Jan 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 166 hospitalized NSCLC patients with COVID-19 showing no significant difference in mortality with paxlovid or azvudine in univariate analysis.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details.
Study covers paxlovid and azvudine.
|
risk of death, 13.0% higher, OR 1.13, p = 0.85, treatment 42, control 124, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Peng et al., 31 Jan 2024, retrospective, China, peer-reviewed, 8 authors, study period 12 December, 2022 - 15 January, 2023.
Contact: lzh202021@126.com, clmedic@126.com.
Impact of Anti-angiogenic Drugs on Severity of COVID-19 in Patients with Non-Small Cell Lung Cancer
Technology in Cancer Research & Treatment, doi:10.1177/15330338241248573
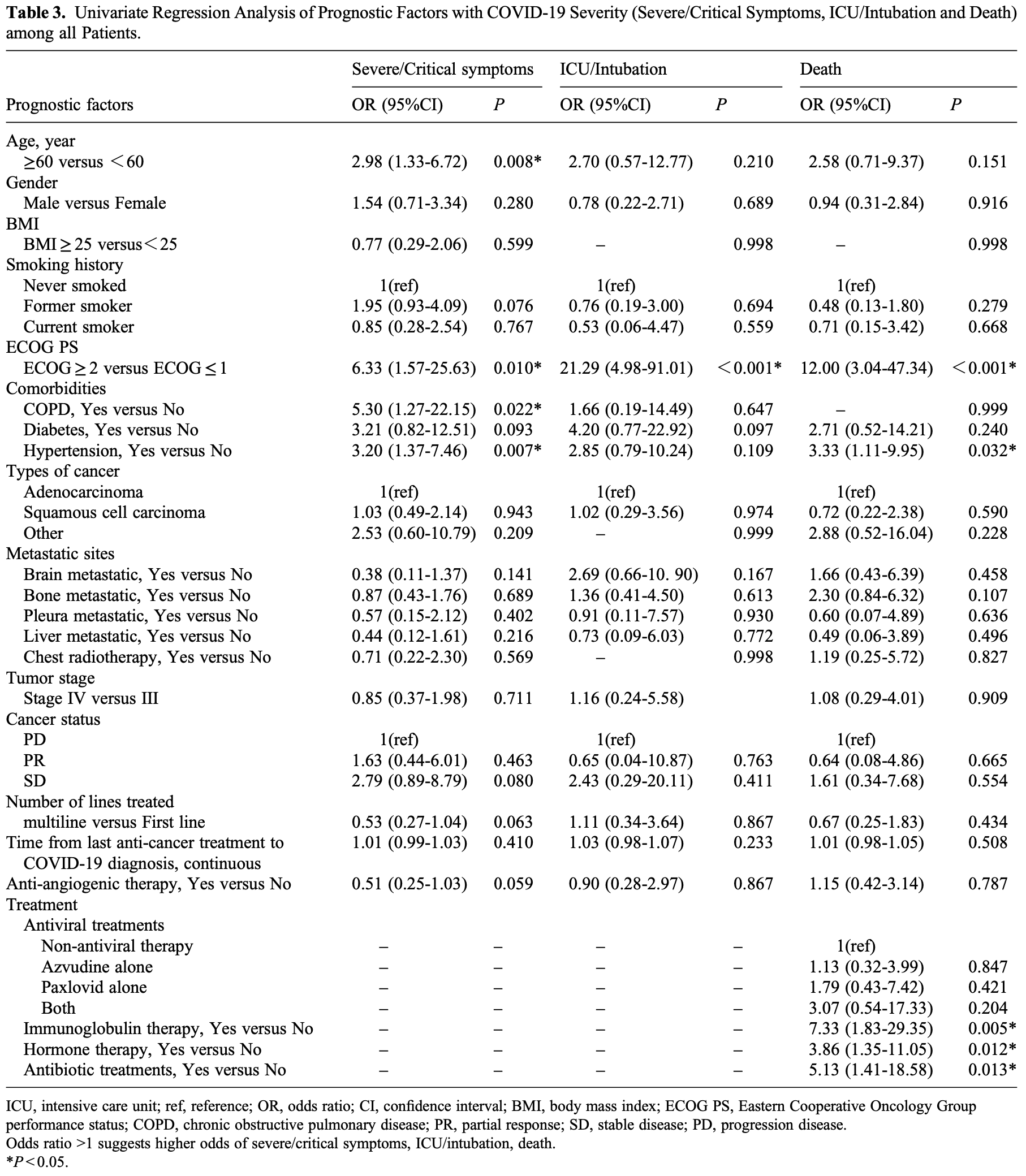
Introduction: The 2019 coronavirus disease (COVID-19) pandemic has reshaped oncology practice, but the impact of antiangiogenic drugs on the severity of COVID-19 in patients with non-small cell lung cancer (NSCLC) remains unclear. Patients and Methods: We carried out a retrospective study involving 166 consecutive patients with NSCLC who were positive for COVID-19, aiming to determine the effects of anti-angiogenic drugs on disease severity, as defined by severe/critical symptoms, intensive care unit (ICU) admission/intubation, and mortality outcomes. Risk factors were identified using univariate and multivariate logistic regression models. Results: Of the participants, 73 had been administered anti-angiogenic drugs (termed the anti-angiogenic therapy (AT) group), while 93 had not (non-AT group). Comparative analyses showed no significant disparity in the rates of severe/ critical symptoms (21.9% vs 35.5%, P = 0.057), ICU admission/intubation (6.8% vs 7.5%, P = 0.867), or death (11.0% vs 9.7%, P = 0.787) between these two groups. However, elevated risk factors for worse outcomes included age ≥ 60 (odds ratio (OR): 2.52, 95% confidence interval (CI): 1.07-5.92), Eastern Cooperative Oncology Group performance status of 2 or higher (OR: 21.29, 95% CI: 4.98-91.01), chronic obstructive pulmonary disease (OR: 7.25, 95% CI: 1.65-31.81), hypertension (OR: 2.98, 95% CI: 1.20-7.39), and use of immunoglobulin (OR: 5.26, 95% CI: 1.06-26.25). Conclusion: Our data suggests that the use of anti-angiogenic drugs may not exacerbate COVID-19 severity in NSCLC patients, indicating their potential safe application even during the pandemic period.
Author Contributions ZL and LC researched literature and conceived the study. JC, XD, XZ, and YL were involved in protocol development, gaining ethical approval, patient recruitment, and data analysis. SP and HH wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of Conflicting Interests The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research Ethics and Patient Consent The studies involving human participants were reviewed and approved by the research ethics committee of the First Affiliated Hospital of Nanchang University, approval/reference number IIT2023131, with an exemption from informed consent. It was performed in line with the guidelines of the Declaration of Helsinki (revised 2013). This was a retrospective clinical study, and statistical analysis of aggregated, deidentified data did not require specific consent. For this study, the raw data were first extracted from HIS, and patients'
ORCID iD Zhihui Lu https://orcid.org/0009-0008-2131-4443
Supplemental Material Supplemental material for this article is available online.
References
Addeo, Friedlaender, Cancer and COVID-19: unmasking their ties, Cancer Treat Rev
Ali, Elshafei, Saad, Clinical outcomes of intravenous immunoglobulin therapy in COVID-19 related acute respiratory distress syndrome: a retrospective cohort study, BMC Pulm Med
Ascha, Wang, Kumthekar, Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases, Sci Rep
Besse, Moulec, Mazières, Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, phase II study, Clin Cancer Res
Brar, Pinheiro, Shusterman, COVID-19 severity and outcomes in patients with cancer: a matched cohort study, J Clin Oncol
Calabrò, Rossi, Covre, COVID and lung cancer, Curr Oncol Rep
Calles, Aparicio, Alva, Outcomes of COVID-19 in patients with lung cancer treated in a tertiary hospital in Madrid, Front Oncol
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Dai, Liu, Liu, Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak, Cancer Discov
Feng, Wang, Jing, Recombinant human endostatin combined with chemotherapy for advanced squamous cell lung cancer: a meta-analysis, World J Surg Oncol
Fließer, Birnhuber, Marsh, Dysbalance of ACE2 levels -a possible cause for severe COVID-19 outcome in COPD, J Pathol Clin Res
Fu, Huang, Shang, Efficacy and safety of immune checkpoint inhibitors combined with recombinant human endostatin and chemotherapy as the first-line treatment of advanced non-small-cell lung cancer, Future Oncol
Ganti, Fillmore, Bihn, Risk factors of SARS-CoV-2 infection and complications from COVID-19 in lung cancer patients, Int J Clin Oncol
Garassino, Whisenant, Huang, COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study, Lancet Oncol
Grivas, Khaki, Wise-Draper, Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and cancer consortium, Ann Oncol
Haineala, Zgura, Badiu, Lung cancer, COVID-19 infections and chemotherapy, Vivo
Hainsworth, Fang, Huang, BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/ paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer, J Thorac Oncol
Han, Li, Wang, Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial, JAMA Oncol
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Huang, Chen, Recombinant human endostatin improves antitumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma, J Cancer Res Clin Oncol
Huang, Zhong, Zhu, Immunotherapy combined with rh-endostatin improved clinical outcomes over immunotherapy plus chemotherapy for second-line treatment of advanced NSCLC, Front Oncol
Itac, INSIGHT 013) Study Group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial, Lancet
Jiang, Qiao, Updating advances on recombinant human endostatin combined with radiotherapy for non-small cell lung cancer with brain metastasis, Transl Lung Cancer Res
Johansen, Mahbub, Idrees, Increased SARS-CoV-2 infection, protease, and inflammatory responses in chronic obstructive pulmonary disease primary bronchial epithelial cells defined with single-cell RNA sequencing, Am J Respir Crit Care Med
Johnson, Fehrenbacher, Novotny, Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer, J Clin Oncol
Kuba, Yamaguchi, Penninger, Angiotensin-converting enzyme 2 (ACE2) in the pathogenesis of ARDS in COVID-19, Front Immunol
Kuderer, Choueiri, Shah, Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study, Lancet
Lee, Cazier, Angelis, COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study, Lancet
Lei, Yang, Zhou, Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis, Lung Cancer
Letko, Marzi, Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses, Nat Microbiol
Leung, Yang, Tam, ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19, Eur Respir J
Li, Zhang, Zhuo, The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases, Pharmacol Res
Li, Zheng, Wei, Effects of recombinant human endostatin and its synergy with cisplatin on circulating endothelial cells and tumor vascular normalization in A549 xenograft murine model, J Cancer Res Clin Oncol
Lin, Ye, Liu, Endostar inhibits hypoxia-induced cell proliferation and migration via the hypoxia-inducible factor-1α/ vascular endothelial growth factor pathway in vitro, Mol Med Rep
Ling, Yang, Lu, Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells, Biochem Biophys Res Commun
Lièvre, Turpin, Ray-Coquard, Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19), Eur J Cancer
Luo, Rizvi, Egger, Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers, Cancer Discov
Luo, Rizvi, Preeshagul, COVID-19 in patients with lung cancer, Ann Oncol
Lv, Pei, Zhao, Safety and efficacy of nivolumab plus recombinant human endostatin in previously treated advanced non-small-cell lung cancer, Transl Lung Cancer Res
Maki, Takada, Taniguchi, Immune checkpoint inhibitor therapy and elevated levels of C-reactive protein associated with COVID-19 aggravation in patients with lung cancer, J Pharm Health Care Sci
Mccoy, Wambier, Vano-Galvan, Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19?, J Cosmet Dermatol
Miyashita, Mikami, Chopra, Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City, Ann Oncol
Nie, Dai, Wu, Clinical characteristics and risk factors for in-hospital mortality of lung cancer patients with COVID-19: a multicenter, retrospective, cohort study, Thorac Cancer
Peng, Xiang, Xu, Redistribution and activation of CD16brightCD56dim NK cell subset to fight against Omicron Subvariant BA.2 after COVID-19 vaccination, Microorganisms
Peng, Xu, Wang, Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models, PLoS One
Pradhan, Olsson, Sex differences in severity and mortality from COVID-19: are males more vulnerable?, Biol Sex Differ
Pu, Wang, Liu, Rh-endostatin plus camrelizumab and chemotherapy in first-line treatment of advanced non-small cell lung cancer: a multicenter retrospective study, Cancer Med
Robilotti, Babady, Mead, Determinants of COVID-19 disease severity in patients with cancer, Nat Med
Salah, Khamees, Tumor angiogenesis: current challenges and therapeutic opportunities, Cancer Treat Res Commun
Sandler, Johnson, Herbst, Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer, Clin Cancer Res
Socinski, Jotte, Cappuzzo, Atezolizumab for firstline treatment of metastatic nonsquamous NSCLC, N Engl J Med
Sun, Niu, Du, Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors, J Hematol Oncol
Sun, Wang, Liu, Long-term results of a randomized, double-blind, and placebo-controlled phase III trial: endostar (rh-endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non-small cell lung cancer, Thorac Cancer
Tan, Yang, Prevention and control strategies for the diagnosis and treatment of cancer patients during the COVID-19 pandemic, Br J Cancer
Tang, Guo, Zhang, Greater efficacy of chemotherapy plus bevacizumab compared to chemo-and targeted therapy alone on non-small cell lung cancer patients with brain metastasis, Oncotarget
Thomas, Gurvich, Kulkarni, Sex differences and COVID-19, Adv Exp Med Biol
Ulhaq, Soraya, Zambrano, Sexual dimorphism in SARS-COV-2 infection, Acta Endocrinol (Buchar)
Viana, Moyo, Amoako, Rapid epidemic expansion of the SARS-CoV-2 omicron variant in Southern Africa, Nature
Von Elm, Altman, Egger, The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Ann Intern Med
Walls, Park, Tortorici, Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Xie, Cao, Dong, Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19, J Infect
Xu, Mao, Chen, Endostar, a modified recombinant human endostatin, suppresses angiogenesis through inhibition of Wnt/β-catenin signaling pathway, PLoS One
Xu, Wang, Zhang, As the SARS-CoV-2 virus evolves, should Omicron subvariant BA.2 be subjected to quarantine, or should we learn to live with it?, Front Public Health
Xu, Ye, Li, Endostar, a recently introduced recombinant human endostatin, inhibits proliferation and migration through regulating growth factors, adhesion factors and inflammatory mediators in choroid-retinal endothelial cells, Mol Biol (Mosk)
Yang, Chai, Yu, Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants, Cancer Biol Med
Zhang, You, Liu, Osimertinib combined with bevacizumab as the first-line treatment in non-small cell lung cancer patients with brain metastasis harboring epidermal growth factor receptor mutations, Thorac Cancer
Zhou, He, Li, The safety and effectiveness of Bevacizumab in the treatment of nonsquamous non-small-cell lung cancer: a meta-analysis of randomized controlled trials, Biomed Res Int
DOI record:
{
"DOI": "10.1177/15330338241248573",
"ISSN": [
"1533-0346",
"1533-0338"
],
"URL": "http://dx.doi.org/10.1177/15330338241248573",
"abstract": "<jats:p> Introduction: The 2019 coronavirus disease (COVID-19) pandemic has reshaped oncology practice, but the impact of anti-angiogenic drugs on the severity of COVID-19 in patients with non-small cell lung cancer (NSCLC) remains unclear. Patients and Methods: We carried out a retrospective study involving 166 consecutive patients with NSCLC who were positive for COVID-19, aiming to determine the effects of anti-angiogenic drugs on disease severity, as defined by severe/critical symptoms, intensive care unit (ICU) admission/intubation, and mortality outcomes. Risk factors were identified using univariate and multivariate logistic regression models. Results: Of the participants, 73 had been administered anti-angiogenic drugs (termed the anti-angiogenic therapy (AT) group), while 93 had not (non-AT group). Comparative analyses showed no significant disparity in the rates of severe/critical symptoms (21.9% vs 35.5%, P = 0.057), ICU admission/intubation (6.8% vs 7.5%, P = 0.867), or death (11.0% vs 9.7%, P = 0.787) between these two groups. However, elevated risk factors for worse outcomes included age ≥ 60 (odds ratio (OR): 2.52, 95% confidence interval (CI): 1.07-5.92), Eastern Cooperative Oncology Group performance status of 2 or higher (OR: 21.29, 95% CI: 4.98-91.01), chronic obstructive pulmonary disease (OR: 7.25, 95% CI: 1.65-31.81), hypertension (OR: 2.98, 95% CI: 1.20-7.39), and use of immunoglobulin (OR: 5.26, 95% CI: 1.06-26.25). Conclusion: Our data suggests that the use of anti-angiogenic drugs may not exacerbate COVID-19 severity in NSCLC patients, indicating their potential safe application even during the pandemic period. </jats:p>",
"alternative-id": [
"10.1177/15330338241248573"
],
"author": [
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Peng",
"given": "Sujuan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Huang",
"given": "Hongxiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Chen",
"given": "Jinhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Ding",
"given": "Xinjing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Zhu",
"given": "Xie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Liu",
"given": "Yangyang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"family": "Chen",
"given": "Li",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0008-2131-4443",
"affiliation": [
{
"name": "Department of Oncology, The First Affiliated Hospital of Nanchang University, Jiangxi, China"
}
],
"authenticated-orcid": false,
"family": "Lu",
"given": "Zhihui",
"sequence": "additional"
}
],
"container-title": "Technology in Cancer Research & Treatment",
"container-title-short": "Technol Cancer Res Treat",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
24
]
],
"date-time": "2024-04-24T15:31:05Z",
"timestamp": 1713972665000
},
"deposited": {
"date-parts": [
[
2024,
4,
24
]
],
"date-time": "2024-04-24T15:32:15Z",
"timestamp": 1713972735000
},
"indexed": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T00:27:06Z",
"timestamp": 1714004826902
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/15330338241248573",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/15330338241248573",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/15330338241248573",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
24
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/S1470-2045(20)30314-4",
"author": "Garassino MC",
"doi-asserted-by": "crossref",
"first-page": "914",
"issue": "7",
"journal-title": "Lancet Oncol",
"key": "bibr1-15330338241248573",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"author": "Chen N",
"doi-asserted-by": "crossref",
"first-page": "507",
"issue": "10223",
"journal-title": "Lancet",
"key": "bibr2-15330338241248573",
"volume": "395",
"year": "2020"
},
{
"key": "bibr3-15330338241248573",
"unstructured": "World Health Organization Data. WHO COVID-19 dashboard.[2023 November 22]. Available from:https://data.who.int/dashboards/covid19/cases?n=o."
},
{
"DOI": "10.1007/s11912-021-01125-8",
"author": "Calabrò L",
"doi-asserted-by": "crossref",
"first-page": "134",
"issue": "11",
"journal-title": "Curr Oncol Rep",
"key": "bibr4-15330338241248573",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04411-y",
"author": "Viana R",
"doi-asserted-by": "crossref",
"first-page": "679",
"issue": "7902",
"journal-title": "Nature",
"key": "bibr5-15330338241248573",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1158/2159-8290.CD-20-0422",
"author": "Dai M",
"doi-asserted-by": "crossref",
"first-page": "783",
"issue": "6",
"journal-title": "Cancer Discov",
"key": "bibr6-15330338241248573",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.007",
"author": "Luo J",
"doi-asserted-by": "crossref",
"first-page": "1386",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "bibr7-15330338241248573",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1158/2159-8290.CD-20-0596",
"author": "Luo J",
"doi-asserted-by": "crossref",
"first-page": "1121",
"issue": "8",
"journal-title": "Cancer Discov",
"key": "bibr8-15330338241248573",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1111/1759-7714.13710",
"author": "Nie L",
"doi-asserted-by": "crossref",
"first-page": "57",
"issue": "1",
"journal-title": "Thorac Cancer",
"key": "bibr9-15330338241248573",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s40780-022-00259-6",
"author": "Maki M",
"doi-asserted-by": "crossref",
"first-page": "27",
"issue": "1",
"journal-title": "J Pharm Health Care Sci",
"key": "bibr10-15330338241248573",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1007/s10147-023-02311-3",
"author": "Ganti AK",
"doi-asserted-by": "crossref",
"first-page": "531",
"issue": "4",
"journal-title": "Int J Clin Oncol",
"key": "bibr11-15330338241248573",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.3389/fonc.2020.01777",
"author": "Calles A",
"doi-asserted-by": "crossref",
"first-page": "1777",
"journal-title": "Front Oncol",
"key": "bibr12-15330338241248573",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.ctrv.2020.102041",
"author": "Addeo A",
"doi-asserted-by": "crossref",
"first-page": "102041",
"journal-title": "Cancer Treat Rev",
"key": "bibr13-15330338241248573",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.21873/invivo.12450",
"author": "Haineala B",
"doi-asserted-by": "crossref",
"first-page": "1877",
"issue": "3",
"journal-title": "In Vivo",
"key": "bibr14-15330338241248573",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1158/1078-0432.CCR-040023",
"author": "Sandler AB",
"doi-asserted-by": "crossref",
"first-page": "4258s",
"issue": "12",
"journal-title": "Clin Cancer Res",
"key": "bibr15-15330338241248573",
"volume": "10",
"year": "2004"
},
{
"author": "Zhou Y",
"first-page": "5537899",
"journal-title": "Biomed Res Int",
"key": "bibr16-15330338241248573",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2007.06.155",
"author": "Ling Y",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "1",
"journal-title": "Biochem Biophys Res Commun",
"key": "bibr17-15330338241248573",
"volume": "361",
"year": "2007"
},
{
"author": "Xu X",
"issue": "9",
"journal-title": "PLoS One",
"key": "bibr18-15330338241248573",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1186/s12957-021-02161-1",
"author": "Feng L",
"doi-asserted-by": "crossref",
"first-page": "64",
"issue": "1",
"journal-title": "World J Surg Oncol",
"key": "bibr19-15330338241248573",
"volume": "19",
"year": "2021"
},
{
"author": "Xu W",
"first-page": "664",
"issue": "4",
"journal-title": "Mol Biol (Mosk)",
"key": "bibr20-15330338241248573",
"volume": "44",
"year": "2010"
},
{
"DOI": "10.3892/mmr.2014.3131",
"author": "Lin K",
"doi-asserted-by": "crossref",
"first-page": "3780",
"issue": "5",
"journal-title": "Mol Med Rep",
"key": "bibr21-15330338241248573",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1007/s00432-010-0770-6",
"author": "Huang G",
"doi-asserted-by": "crossref",
"first-page": "1201",
"issue": "8",
"journal-title": "J Cancer Res Clin Oncol",
"key": "bibr22-15330338241248573",
"volume": "136",
"year": "2010"
},
{
"author": "Peng F",
"issue": "4",
"journal-title": "PLoS One",
"key": "bibr23-15330338241248573",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1007/s00432-012-1189-z",
"author": "Li N",
"doi-asserted-by": "crossref",
"first-page": "1131",
"issue": "7",
"journal-title": "J Cancer Res Clin Oncol",
"key": "bibr24-15330338241248573",
"volume": "138",
"year": "2012"
},
{
"DOI": "10.1186/s13045-016-0332-8",
"author": "Sun Y",
"doi-asserted-by": "crossref",
"first-page": "105",
"issue": "1",
"journal-title": "J Hematol Oncol",
"key": "bibr25-15330338241248573",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1001/jamaoncol.2018.3039",
"author": "Han B",
"doi-asserted-by": "crossref",
"first-page": "1569",
"issue": "11",
"journal-title": "JAMA Oncol",
"key": "bibr26-15330338241248573",
"volume": "4",
"year": "2018"
},
{
"DOI": "10.1016/j.ctarc.2021.100422",
"author": "Al-Ostoot FH",
"doi-asserted-by": "crossref",
"first-page": "100422",
"journal-title": "Cancer Treat Res Commun",
"key": "bibr27-15330338241248573",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1111/1759-7714.12050",
"author": "Sun Y",
"doi-asserted-by": "crossref",
"first-page": "440",
"issue": "4",
"journal-title": "Thorac Cancer",
"key": "bibr28-15330338241248573",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1056/NEJMoa1716948",
"author": "Socinski MA",
"doi-asserted-by": "crossref",
"first-page": "2288",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "bibr29-15330338241248573",
"volume": "378",
"year": "2018"
},
{
"DOI": "10.1002/cam4.5526",
"author": "Pu X",
"doi-asserted-by": "crossref",
"first-page": "7724",
"issue": "7",
"journal-title": "Cancer Med",
"key": "bibr30-15330338241248573",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.21037/tlcr-22-49",
"author": "Lv W",
"doi-asserted-by": "crossref",
"first-page": "201",
"issue": "2",
"journal-title": "Transl Lung Cancer Res",
"key": "bibr31-15330338241248573",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3389/fonc.2023.1137224",
"author": "Huang H",
"doi-asserted-by": "crossref",
"first-page": "1137224",
"journal-title": "Front Oncol",
"key": "bibr32-15330338241248573",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.2217/fon-2022-0861",
"author": "Fu S",
"doi-asserted-by": "crossref",
"first-page": "147",
"issue": "2",
"journal-title": "Future Oncol",
"key": "bibr33-15330338241248573",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.7326/0003-4819-147-8-200710160-00010",
"author": "von Elm E",
"doi-asserted-by": "crossref",
"first-page": "573",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "bibr34-15330338241248573",
"volume": "147",
"year": "2007"
},
{
"key": "bibr35-15330338241248573",
"unstructured": "National Health Commission of the People's Republic of China. The 10th edition of the 2019 Novel Coronavirus Disease (COVID-19) infection diagnosis and treatmen criteria.[ 2023 January 5]. Available from:http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=32de5b2ff9bf4eaa88e75bdf7223a65a."
},
{
"DOI": "10.1097/JTO.0b013e3181f94ad4",
"author": "Hainsworth JD",
"doi-asserted-by": "crossref",
"first-page": "109",
"issue": "1",
"journal-title": "J Thorac Oncol",
"key": "bibr36-15330338241248573",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1200/JCO.2004.11.022",
"author": "Johnson DH",
"doi-asserted-by": "crossref",
"first-page": "2184",
"issue": "11",
"journal-title": "J Clin Oncol",
"key": "bibr37-15330338241248573",
"volume": "22",
"year": "2004"
},
{
"DOI": "10.1038/s41598-019-54513-3",
"author": "Ascha MS",
"doi-asserted-by": "crossref",
"first-page": "17792",
"issue": "1",
"journal-title": "Sci Rep",
"key": "bibr38-15330338241248573",
"volume": "9",
"year": "2019"
},
{
"author": "Jiang XD",
"first-page": "84",
"issue": "1",
"journal-title": "Transl Lung Cancer Res",
"key": "bibr39-15330338241248573",
"volume": "1",
"year": "2012"
},
{
"DOI": "10.1111/1759-7714.14880",
"author": "Zhang L",
"doi-asserted-by": "crossref",
"first-page": "1355",
"issue": "15",
"journal-title": "Thorac Cancer",
"key": "bibr40-15330338241248573",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.18632/oncotarget.6184",
"author": "Tang N",
"doi-asserted-by": "crossref",
"first-page": "3635",
"issue": "3",
"journal-title": "Oncotarget",
"key": "bibr41-15330338241248573",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1158/1078-0432.CCR-14-2082",
"author": "Besse B",
"doi-asserted-by": "crossref",
"first-page": "1896",
"issue": "8",
"journal-title": "Clin Cancer Res",
"key": "bibr42-15330338241248573",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.3390/microorganisms11040940",
"author": "Peng H",
"doi-asserted-by": "crossref",
"first-page": "940",
"issue": "4",
"journal-title": "Microorganisms",
"key": "bibr43-15330338241248573",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.3389/fpubh.2022.1039123",
"author": "Xu R",
"doi-asserted-by": "crossref",
"first-page": "1039123",
"journal-title": "Front Public Health",
"key": "bibr44-15330338241248573",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.lungcan.2021.05.002",
"author": "Lei H",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "Lung Cancer",
"key": "bibr45-15330338241248573",
"volume": "157",
"year": "2021"
},
{
"DOI": "10.20892/j.issn.2095-3941.2020.0559",
"author": "Yang L",
"doi-asserted-by": "crossref",
"first-page": "298",
"issue": "1",
"journal-title": "Cancer Biol Med",
"key": "bibr46-15330338241248573",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2021.02.024",
"author": "Grivas P",
"doi-asserted-by": "crossref",
"first-page": "787",
"issue": "6",
"journal-title": "Ann Oncol",
"key": "bibr47-15330338241248573",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31187-9",
"author": "Kuderer NM",
"doi-asserted-by": "crossref",
"first-page": "1907",
"issue": "10241",
"journal-title": "Lancet",
"key": "bibr48-15330338241248573",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41416-020-0854-2",
"author": "Tan J",
"doi-asserted-by": "crossref",
"first-page": "5",
"issue": "1",
"journal-title": "Br J Cancer",
"key": "bibr49-15330338241248573",
"volume": "123",
"year": "2020"
},
{
"DOI": "10.1016/j.ejca.2020.09.035",
"author": "Lièvre A",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Eur J Cancer",
"key": "bibr50-15330338241248573",
"volume": "141",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0979-0",
"author": "Robilotti EV",
"doi-asserted-by": "crossref",
"first-page": "1218",
"issue": "8",
"journal-title": "Nat Med",
"key": "bibr51-15330338241248573",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.04.006",
"author": "Miyashita H",
"doi-asserted-by": "crossref",
"first-page": "1088",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "bibr52-15330338241248573",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1200/JCO.20.01580",
"author": "Brar G",
"doi-asserted-by": "crossref",
"first-page": "3914",
"issue": "33",
"journal-title": "J Clin Oncol",
"key": "bibr53-15330338241248573",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31173-9",
"author": "Lee LY",
"doi-asserted-by": "crossref",
"first-page": "1919",
"issue": "10241",
"journal-title": "Lancet",
"key": "bibr54-15330338241248573",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "Hoffmann M",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "bibr55-15330338241248573",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0688-y",
"author": "Letko M",
"doi-asserted-by": "crossref",
"first-page": "562",
"issue": "4",
"journal-title": "Nat Microbiol",
"key": "bibr56-15330338241248573",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"author": "Walls AC",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "2",
"journal-title": "Cell",
"key": "bibr57-15330338241248573",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00688-2020",
"author": "Leung JM",
"doi-asserted-by": "crossref",
"first-page": "2000688",
"issue": "5",
"journal-title": "Eur Respir J",
"key": "bibr58-15330338241248573",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1002/cjp2.224",
"author": "Fließer E",
"doi-asserted-by": "crossref",
"first-page": "446",
"issue": "5",
"journal-title": "J Pathol Clin Res",
"key": "bibr59-15330338241248573",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.732690",
"author": "Kuba K",
"doi-asserted-by": "crossref",
"first-page": "732690",
"journal-title": "Front Immunol",
"key": "bibr60-15330338241248573",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202108-1901OC",
"author": "Johansen MD",
"doi-asserted-by": "crossref",
"first-page": "712",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "bibr61-15330338241248573",
"volume": "206",
"year": "2022"
},
{
"DOI": "10.1016/j.phrs.2017.06.005",
"author": "Li XC",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Pharmacol Res",
"key": "bibr62-15330338241248573",
"volume": "125",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(22)00101-5",
"author": "ITAC (INSIGHT 013) Study Group.",
"doi-asserted-by": "crossref",
"first-page": "530",
"issue": "10324",
"journal-title": "Lancet",
"key": "bibr63-15330338241248573",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1186/s12890-021-01717-x",
"author": "Ali HS",
"doi-asserted-by": "crossref",
"first-page": "354",
"issue": "1",
"journal-title": "BMC Pulm Med",
"key": "bibr64-15330338241248573",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.03.044",
"author": "Xie Y",
"doi-asserted-by": "crossref",
"first-page": "318",
"issue": "2",
"journal-title": "J Infect",
"key": "bibr65-15330338241248573",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1111/jocd.13455",
"author": "McCoy J",
"doi-asserted-by": "crossref",
"first-page": "1542",
"issue": "7",
"journal-title": "J Cosmet Dermatol",
"key": "bibr66-15330338241248573",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.4183/aeb.2020.522",
"author": "Ulhaq ZS",
"doi-asserted-by": "crossref",
"first-page": "522",
"issue": "4",
"journal-title": "Acta Endocrinol (Buchar)",
"key": "bibr67-15330338241248573",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1007/978-3-030-71697-4_6",
"author": "Thomas N",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Adv Exp Med Biol",
"key": "bibr68-15330338241248573",
"volume": "1327",
"year": "2021"
},
{
"DOI": "10.1186/s13293-020-00330-7",
"author": "Pradhan A",
"doi-asserted-by": "crossref",
"first-page": "53",
"issue": "1",
"journal-title": "Biol Sex Differ",
"key": "bibr69-15330338241248573",
"volume": "11",
"year": "2020"
}
],
"reference-count": 69,
"references-count": 69,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/15330338241248573"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Impact of Anti-angiogenic Drugs on Severity of COVID-19 in Patients with Non-Small Cell Lung Cancer",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "23"
}
peng4
