Comparative effectiveness of antiviral treatment on household transmission of SARS-CoV-2: a retrospective cohort study using administrative data
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-11651-6, Sep 2025
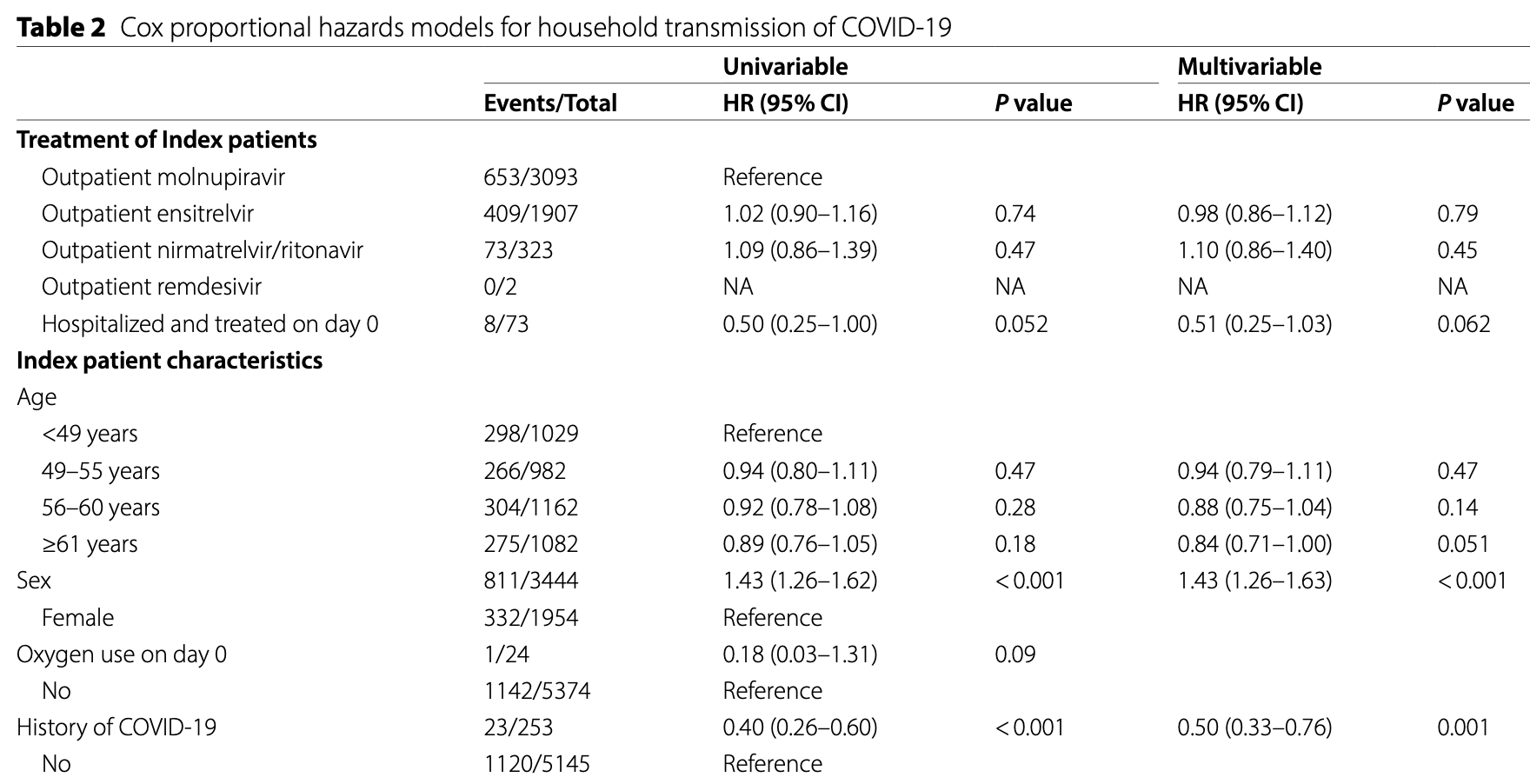
Retrospective 5,398 married couples in Japan showing no significant difference in household transmission rates between molnupiravir, ensitrelvir, and paxlovid. Hospitalized patients receiving antivirals showed a trend toward lower transmission rates compared to outpatients, likely due to physical isolation. Prior COVID-19 infection in either spouse reduced transmission risk by 50-69%. The study used administrative claims data which may underestimate true transmission rates since asymptomatic or mild cases might not seek medical care.
No adjusted results are provided for antiviral use vs. non-use, however authors note transmission for 30.4% of treated households vs. 24.0% for untreated households (for day 0-7, different to subsequent analysis).
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Study covers ensitrelvir, paxlovid, and molnupiravir.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Ikeuchi et al., 29 Sep 2025, retrospective, Japan, peer-reviewed, 9 authors, study period 1 April, 2023 - 31 August, 2023.
Contact: kikeuchi@g.ecc.u-tokyo.ac.jp.
Comparative effectiveness of antiviral treatment on household transmission of SARS-CoV-2: a retrospective cohort study using administrative data
BMC Infectious Diseases, doi:10.1186/s12879-025-11651-6
Background Antiviral treatment reduces influenza transmission and differs in effectiveness among agents. Although SARS-CoV-2 antivirals lower viral shedding, their role in preventing secondary household transmission and the differences between agents remain unclear.
Methods We conducted a retrospective cohort study using the JMDC administrative claims database in Japan. The study included married-couple households between 1 April and 31 August 2023, when the Omicron XBB variant was predominant. Households in which at least one person had been diagnosed with Coronavirus Disease 2019 (COVID-19) were included. We excluded households if the index patient did not receive antiviral treatment on day 0, or the spouse was diagnosed on day 0 or 1. The primary outcome was subsequent infection in the spouse by day 7. Cox proportional hazards models were used to estimate hazard ratios (HRs), after adjusting for potential confounders.
Results Of the 326,827 married-couple households, 5,398 met the inclusion criteria. Among them, 1,143 households (21.2%) experienced presumed secondary transmission by day 7. The cumulative transmission rate, estimated using the Kaplan-Meier method, was lower among hospitalized patients (n = 73, 11.0%, 95% confidence interval [CI]: 5.7-20.8%) than among outpatients (n = 5,325, 21.5%, 95% CI: 20.4-22.6%, p = 0.035). Transmission rates did not significantly differ among the outpatient antiviral groups: molnupiravir (n = 3,093, 21.3%, 95% CI: 19.9-22.8%), ensitrelvir (n = 1,907, 21.6%, 95% CI: 19.8-23.6%), and nirmatrelvir/ritonavir (n = 323, 22.8%, 95% CI: 18.6-27.8%, p = 0.74). In multivariable Cox analysis, male sex (adjusted HR 1.43, 95% CI: 1.26-1.63; p < 0.001), history of COVID-19 in the index patient (adjusted HR 0.50, 95% CI: 0.33-0.76; p = 0.001), and history of COVID-19 in the partner (adjusted HR 0.31, 95% CI: 0.21-0.45; p < 0.001) were significantly associated with transmission risk. Hospitalization tended to be associated with a lower risk of transmission (adjusted HR, 0.51; 95% CI, 0.25-1.03; p = 0.062).
Abbreviations
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 1 8 6 / s 1 2 8 7 9 -0 2 5 -1 1 6 5 1 -6.
Supplementary Material 1 Authors' contributions KI, MS, and TT conceived the study. KI performed the data analysis and drafted the manuscript. KI and MS conducted the statistical analyses. KO, AK, SM, TK, YA, and HY contributed to the data interpretation and critically revised the manuscript. All the authors (KI, MS, TT, KO, AK, SM, TK, YA, and HY) reviewed the manuscript for important intellectual content and approved its final version.
Declarations Ethics approval and consent to participate This study was approved by the Research Ethics Committee of the University of Tokyo (approval number 2024216NIe). The Ethics committee of University of Tokyo waived the requirement for informed consent because the study used fully anonymized administrative data that were not individually identifiable. Data confidentiality was strictly maintained in accordance with the Declaration of Helsinki.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Alpizar, Accini, Anderson, Eysa, Medina-Piñón et al., Molnupiravir for intra-household prevention of COVID-19: the MOVe-AHEAD randomized, placebo-controlled trial, J Infect
Baker, Nakayama, Hegarty, Mcgowan, Teran et al., Household transmission of SARS-CoV-2 in five US jurisdictions: comparison of delta and omicron variants, PLoS ONE
Benchimol, Smeeth, Guttmann, Harron, Moher et al., The reporting of studies conducted using observational routinely-collected health data (RECORD) statement, PLoS Med
Bender, Brandl, Höhle, Buchholz, Zeitlmann, Analysis of asymptomatic and presymptomatic transmission in SARS-CoV-2 outbreak, Germany, Emerg Infect Dis
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Bertakis, Azari, Helms, Callahan, Robbins, Gender differences in the utilization of health care services, J Fam Pract
Cox, Lieber, Wolf, Karimi, Lieberman et al., Comparing molnupiravir and nirmatrelvir/ritonavir efficacy and the effects on SARS-CoV-2 transmission in animal models, Nat Commun
Gao, Liu, Li, Xu, Zhang et al., Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials, Clin Microbiol Infect
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Hammond, Yunis, Fountaine, Luscan, Burr et al., Oral nirmatrelvir-ritonavir as postexposure prophylaxis for Covid-19, N Engl J Med
Han, Park, Jeong, Lee, Park, COVID-19 cluster linked to aerosol transmission of SARS-CoV-2 via floor drains, J Infect Dis
Hashimoto, Saito, Sato, Goda, Mitsutake et al., Indications and classes of outpatient antibiotic prescriptions in Japan: a descriptive study using the National database of electronic health insurance claims, 2012-2015, Int J Infect Dis
Hirotsu, Saisho, Hasegawa, The effect of neuraminidase inhibitors on household transmission in Japanese patients with influenza A and B infection: a prospective, observational study, Influenza Other Respir Viruses
Ikematsu, Hayden, Kawaguchi, Kinoshita, De Jong et al., Baloxavir marboxil for prophylaxis against influenza in household contacts, N Engl J Med
Kaneko, Yano, Itoh, Morita, Kiriyama et al., Association of blood pressure classification using the 2017 American college of cardiology/american heart association blood pressure guideline with risk of heart failure and atrial fibrillation, Circulation
Komeda, Takazono, Hosogaya, Ogura, Fujiwara et al., Comparison of household transmission of influenza virus from index patients treated with Baloxavir Marboxil or neuraminidase inhibitors: A health insurance claims database study, Clin Infect Dis
Lyngse, Mortensen, Denwood, Christiansen, Møller et al., Household transmission of the SARS-CoV-2 Omicron variant in Denmark, Nat Commun
Madewell, Yang, Longini, Jr, Halloran et al., Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis, JAMA Netw Open
Meyerowitz, Richterman, Gandhi, Sax, Transmission of SARS-CoV-2: a review of viral, host, and environmental factors, Ann Intern Med
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis
Ogata, Tanaka, SARS-CoV-2 incubation period during the Omicron BA.5dominant period in Japan, Emerg Infect Dis
Onrust, Lamb, Balfour, None, Rituximab Drugs
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol
Schilling, Jittamala, Watson, Boyd, Luvira et al., Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial, Lancet Infect Dis
Shirata, Ito, Jo, Iwao, Oi et al., Factors associated with the development of bacterial pneumonia related to seasonal influenza virus infection: a study using a large-scale health insurance claim database, Open Forum Infect Dis
Yotsuyanagi, Ohmagari, Doi, Yamato, Bac et al., Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial, JAMA Netw Open
Zhu, Zhang, Li, Yang, Song, A novel coronavirus from patients with pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1186/s12879-025-11651-6",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-025-11651-6",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Antiviral treatment reduces influenza transmission and differs in effectiveness among agents. Although SARS-CoV-2 antivirals lower viral shedding, their role in preventing secondary household transmission and the differences between agents remain unclear.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a retrospective cohort study using the JMDC administrative claims database in Japan. The study included married-couple households between 1 April and 31 August 2023, when the Omicron XBB variant was predominant. Households in which at least one person had been diagnosed with Coronavirus Disease 2019 (COVID-19) were included. We excluded households if the index patient did not receive antiviral treatment on day 0, or the spouse was diagnosed on day 0 or 1. The primary outcome was subsequent infection in the spouse by day 7. Cox proportional hazards models were used to estimate hazard ratios (HRs), after adjusting for potential confounders.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of the 326,827 married-couple households, 5,398 met the inclusion criteria. Among them, 1,143 households (21.2%) experienced presumed secondary transmission by day 7. The cumulative transmission rate, estimated using the Kaplan–Meier method, was lower among hospitalized patients (<jats:italic>n</jats:italic> = 73, 11.0%, 95% confidence interval [CI]: 5.7–20.8%) than among outpatients (<jats:italic>n</jats:italic> = 5,325, 21.5%, 95% CI: 20.4–22.6%, <jats:italic>p</jats:italic> = 0.035). Transmission rates did not significantly differ among the outpatient antiviral groups: molnupiravir (<jats:italic>n</jats:italic> = 3,093, 21.3%, 95% CI: 19.9–22.8%), ensitrelvir (<jats:italic>n</jats:italic> = 1,907, 21.6%, 95% CI: 19.8–23.6%), and nirmatrelvir/ritonavir (<jats:italic>n</jats:italic> = 323, 22.8%, 95% CI: 18.6–27.8%, <jats:italic>p</jats:italic> = 0.74). In multivariable Cox analysis, male sex (adjusted HR 1.43, 95% CI: 1.26–1.63; <jats:italic>p</jats:italic> < 0.001), history of COVID-19 in the index patient (adjusted HR 0.50, 95% CI: 0.33–0.76; <jats:italic>p</jats:italic> = 0.001), and history of COVID-19 in the partner (adjusted HR 0.31, 95% CI: 0.21–0.45; <jats:italic>p</jats:italic> < 0.001) were significantly associated with transmission risk. Hospitalization tended to be associated with a lower risk of transmission (adjusted HR, 0.51; 95% CI, 0.25–1.03; <jats:italic>p</jats:italic> = 0.062).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Household transmission rates were not statistically different among three different outpatient oral antiviral agents. Hospitalization was associated with a trend toward lower transmission rates, possibly due to physical isolation.</jats:p>\n </jats:sec>",
"alternative-id": [
"11651"
],
"article-number": "1213",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 June 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "8 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "29 September 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was approved by the Research Ethics Committee of the University of Tokyo (approval number 2024216NIe). The Ethics committee of University of Tokyo waived the requirement for informed consent because the study used fully anonymized administrative data that were not individually identifiable. Data confidentiality was strictly maintained in accordance with the Declaration of Helsinki."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Ikeuchi",
"given": "Kazuhiko",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saito",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okushin",
"given": "Kazuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arisato",
"given": "Yuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kishida",
"given": "Toshiyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsumoto",
"given": "Shinya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kado",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yotsuyanagi",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsutsumi",
"given": "Takeya",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T19:28:03Z",
"timestamp": 1759174083000
},
"deposited": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T19:28:06Z",
"timestamp": 1759174086000
},
"funder": [
{
"award": [
"JPMJPR23R1"
],
"name": "JST, PRESTO"
}
],
"indexed": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T20:10:27Z",
"timestamp": 1759176627275,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
9,
29
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T00:00:00Z",
"timestamp": 1759104000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T00:00:00Z",
"timestamp": 1759104000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-11651-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-025-11651-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-11651-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
9,
29
]
]
},
"published-online": {
"date-parts": [
[
2025,
9,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"author": "N Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "11651_CR1",
"unstructured": "Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.7326/M20-5008",
"author": "EA Meyerowitz",
"doi-asserted-by": "publisher",
"first-page": "69",
"issue": "1",
"journal-title": "Ann Intern Med",
"key": "11651_CR2",
"unstructured": "Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174(1):69–79.",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab598",
"author": "T Han",
"doi-asserted-by": "publisher",
"first-page": "1554",
"issue": "9",
"journal-title": "J Infect Dis",
"key": "11651_CR3",
"unstructured": "Han T, Park H, Jeong Y, Lee J, Shon E, Park MS, et al. COVID-19 cluster linked to aerosol transmission of SARS-CoV-2 via floor drains. J Infect Dis. 2022;225(9):1554–60.",
"volume": "225",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2309002",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "224",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "11651_CR4",
"unstructured": "Hammond J, Yunis C, Fountaine RJ, Luscan G, Burr AM, Zhang W, et al. Oral nirmatrelvir-ritonavir as postexposure prophylaxis for Covid-19. N Engl J Med. 2024;391(3):224–34.",
"volume": "391",
"year": "2024"
},
{
"DOI": "10.1001/jamanetworkopen.2022.9317",
"author": "ZJ Madewell",
"doi-asserted-by": "publisher",
"first-page": "e229317",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "11651_CR5",
"unstructured": "Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open. 2022;5(4):e229317.",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0313680",
"author": "JM Baker",
"doi-asserted-by": "publisher",
"first-page": "e0313680",
"issue": "1",
"journal-title": "PLoS ONE",
"key": "11651_CR6",
"unstructured": "Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, et al. Household transmission of SARS-CoV-2 in five US jurisdictions: comparison of delta and omicron variants. PLoS ONE. 2025;20(1):e0313680.",
"volume": "20",
"year": "2025"
},
{
"DOI": "10.1038/s41467-022-33328-3",
"author": "FP Lyngse",
"doi-asserted-by": "publisher",
"first-page": "5573",
"issue": "1",
"journal-title": "Nat Commun",
"key": "11651_CR7",
"unstructured": "Lyngse FP, Mortensen LH, Denwood MJ, Christiansen LE, Møller CH, Skov RL, Spiess K, Fomsgaard A, Lassaunière R, Rasmussen M, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. 2022;13(1):5573.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "11651_CR8",
"unstructured": "Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342-9.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2023.04.014",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"first-page": "979",
"issue": "8",
"journal-title": "Clin Microbiol Infect",
"key": "11651_CR9",
"unstructured": "Gao Y, Liu M, Li Z, Xu J, Zhang J, Tian J. Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin Microbiol Infect. 2023;29(8):979–99.",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "11651_CR10",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"author": "H Yotsuyanagi",
"doi-asserted-by": "publisher",
"first-page": "e2354991",
"issue": "2",
"journal-title": "JAMA Netw Open",
"key": "11651_CR11",
"unstructured": "Yotsuyanagi H, Ohmagari N, Doi Y, Yamato M, Bac NH, Cha BK, et al. Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial. JAMA Netw Open. 2024;7(2):e2354991.",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "4",
"journal-title": "N Engl J Med",
"key": "11651_CR12",
"unstructured": "Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386(4):305–15.",
"volume": "386",
"year": "2022"
},
{
"key": "11651_CR13",
"unstructured": "Ministry of Health Labour and Welfare Japan. Clinical Management of Patients with COVID-19: Guide for Medical Practitioners [in Japanese]. [https://www.mhlw.go.jp/content/001248424.pdf, Accessed on date [2025 Jul 18]."
},
{
"DOI": "10.1111/irv.12590",
"author": "N Hirotsu",
"doi-asserted-by": "publisher",
"first-page": "123",
"issue": "2",
"journal-title": "Influenza Other Respir Viruses",
"key": "11651_CR14",
"unstructured": "Hirotsu N, Saisho Y, Hasegawa T. The effect of neuraminidase inhibitors on household transmission in Japanese patients with influenza A and B infection: a prospective, observational study. Influenza Other Respir Viruses. 2019;13(2):123–32.",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1093/cid/ciaa1622",
"author": "T Komeda",
"doi-asserted-by": "publisher",
"first-page": "e859",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "11651_CR15",
"unstructured": "Komeda T, Takazono T, Hosogaya N, Ogura E, Fujiwara M, Miyauchi H, Ajisawa Y, Iwata S, Watanabe H, Honda K, et al. Comparison of household transmission of influenza virus from index patients treated with Baloxavir Marboxil or neuraminidase inhibitors: A health insurance claims database study. Clin Infect Dis. 2021;72(11):e859–67.",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1038/s41467-023-40556-8",
"author": "RM Cox",
"doi-asserted-by": "publisher",
"first-page": "4731",
"issue": "1",
"journal-title": "Nat Commun",
"key": "11651_CR16",
"unstructured": "Cox RM, Lieber CM, Wolf JD, Karimi A, Lieberman NAP, Sticher ZM, Roychoudhury P, Andrews MK, Krueger RE, Natchus MG, et al. Comparing molnupiravir and nirmatrelvir/ritonavir efficacy and the effects on SARS-CoV-2 transmission in animal models. Nat Commun. 2023;14(1):4731.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3201/eid2704.204576",
"author": "JK Bender",
"doi-asserted-by": "publisher",
"first-page": "1159",
"issue": "4",
"journal-title": "Emerg Infect Dis",
"key": "11651_CR17",
"unstructured": "Bender JK, Brandl M, Höhle M, Buchholz U, Zeitlmann N. Analysis of asymptomatic and presymptomatic transmission in SARS-CoV-2 outbreak, Germany, 2020. Emerg Infect Dis. 2021;27(4):1159–63.",
"volume": "27",
"year": "2021"
},
{
"key": "11651_CR18",
"unstructured": "Japan Institute for Health Security. Current Situation of Infection, August 4. 2023. [https://id-info.jihs.go.jp/diseases/sa/covid-19/320/covid19-ab124th-en.html, Accessed on date [13 May]."
},
{
"DOI": "10.3201/eid2903.221360",
"author": "T Ogata",
"doi-asserted-by": "publisher",
"first-page": "595",
"issue": "3",
"journal-title": "Emerg Infect Dis",
"key": "11651_CR19",
"unstructured": "Ogata T, Tanaka H. SARS-CoV-2 incubation period during the Omicron BA.5-dominant period in Japan. Emerg Infect Dis. 2023;29(3):595–8.",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.052624",
"author": "H Kaneko",
"doi-asserted-by": "publisher",
"first-page": "2244",
"issue": "23",
"journal-title": "Circulation",
"key": "11651_CR20",
"unstructured": "Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, Fujiu K, Michihata N, Jo T, Takeda N, et al. Association of blood pressure classification using the 2017 American college of cardiology/american heart association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021;143(23):2244–53.",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofad222",
"author": "M Shirata",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Open Forum Infect Dis",
"key": "11651_CR21",
"unstructured": "Shirata M, Ito I, Jo T, Iwao T, Oi I, Hamao N, et al. Factors associated with the development of bacterial pneumonia related to seasonal influenza virus infection: a study using a large-scale health insurance claim database. Open Forum Infect Dis. 2023;10(5):ofad222.",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.2165/00003495-199958010-00009",
"author": "SV Onrust",
"doi-asserted-by": "publisher",
"first-page": "79",
"issue": "1",
"journal-title": "Rituximab Drugs",
"key": "11651_CR22",
"unstructured": "Onrust SV, Lamb HM, Balfour JA. Rituximab Drugs. 1999;58(1):79–88. discussion 89–90.",
"volume": "58",
"year": "1999"
},
{
"author": "O Puhach",
"first-page": "147",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "11651_CR23",
"unstructured": "Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023;21(3):147–61.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1371/journal.pmed.1001885",
"author": "EI Benchimol",
"doi-asserted-by": "publisher",
"first-page": "e1001885",
"issue": "10",
"journal-title": "PLoS Med",
"key": "11651_CR24",
"unstructured": "Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885.",
"volume": "12",
"year": "2015"
},
{
"DOI": "10.1016/S1473-3099(23)00493-0",
"author": "WHK Schilling",
"doi-asserted-by": "publisher",
"first-page": "36",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "11651_CR25",
"unstructured": "Schilling WHK, Jittamala P, Watson JA, Boyd S, Luvira V, Siripoon T, et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect Dis. 2024;24(1):36–45.",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa1915341",
"author": "H Ikematsu",
"doi-asserted-by": "publisher",
"first-page": "309",
"issue": "4",
"journal-title": "N Engl J Med",
"key": "11651_CR26",
"unstructured": "Ikematsu H, Hayden FG, Kawaguchi K, Kinoshita M, de Jong MD, Lee N, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383(4):309–20.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2023.08.016",
"author": "SA Alpizar",
"doi-asserted-by": "publisher",
"first-page": "392",
"issue": "5",
"journal-title": "J Infect",
"key": "11651_CR27",
"unstructured": "Alpizar SA, Accini J, Anderson DC, Eysa B, Medina-Piñón I, Ohmagari N, et al. Molnupiravir for intra-household prevention of COVID-19: the MOVe-AHEAD randomized, placebo-controlled trial. J Infect. 2023;87(5):392–402.",
"volume": "87",
"year": "2023"
},
{
"author": "KD Bertakis",
"first-page": "147",
"issue": "2",
"journal-title": "J Fam Pract",
"key": "11651_CR28",
"unstructured": "Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147–52.",
"volume": "49",
"year": "2000"
},
{
"DOI": "10.1016/j.ijid.2019.11.009",
"author": "H Hashimoto",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int J Infect Dis",
"key": "11651_CR29",
"unstructured": "Hashimoto H, Saito M, Sato J, Goda K, Mitsutake N, Kitsuregawa M, et al. Indications and classes of outpatient antibiotic prescriptions in Japan: a descriptive study using the National database of electronic health insurance claims, 2012–2015. Int J Infect Dis. 2020;91:1–8.",
"volume": "91",
"year": "2020"
},
{
"key": "11651_CR30",
"unstructured": "Ministry of Health Labour and Welfare Japan. Number of COVID-19 Vaccine Doses Administered. [https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/yobou-sesshu/syukeihou_00002.html?utm_source=chatgpt.com], Accessed on date [May 21]."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-025-11651-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparative effectiveness of antiviral treatment on household transmission of SARS-CoV-2: a retrospective cohort study using administrative data",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}
ikeuchi