Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database
et al., BMJ Open, doi:10.1136/bmjopen-2022-064953, Dec 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
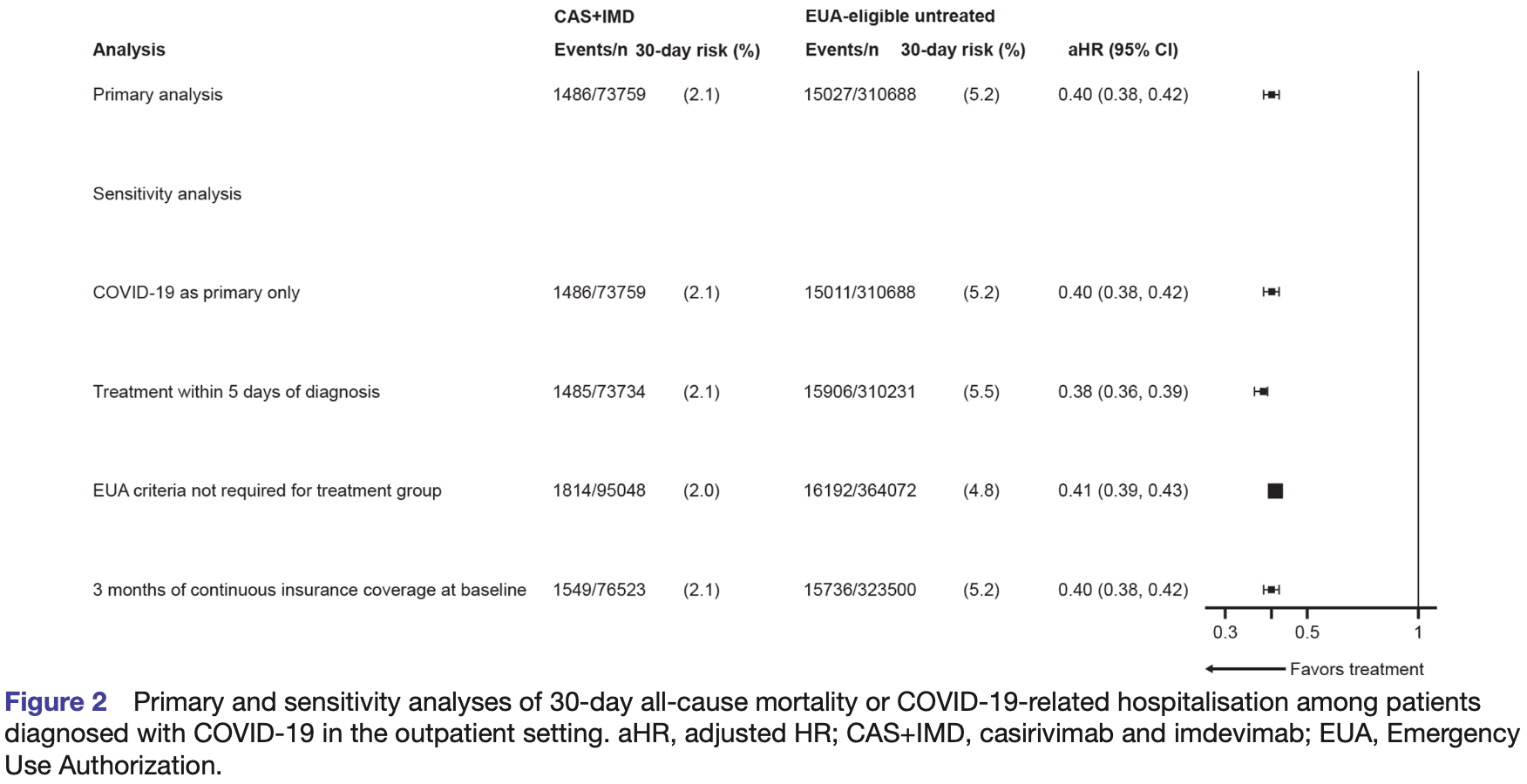
Retrospective 73,759 outpatients treated with casirivimab/imdevimab, showing lower mortality with treatment. This result is subject to potentially substantial confounding by indication - patients with more severe cases may be more likely to receive treatment, and severity information was unavailable in the database.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication possible.
|
risk of death/hospitalization, 60.0% lower, HR 0.40, p < 0.001, NNT 35, propensity score matching, Cox proportional hazards, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Hussein et al., 19 Dec 2022, retrospective, USA, peer-reviewed, 9 authors, study period 1 December, 2020 - 30 September, 2021.
Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database
BMJ Open, doi:10.1136/bmjopen-2022-064953
Objective To assess the real-world effectiveness of casirivimab and imdevimab (CAS+IMD) versus no COVID-19 antibody treatment among patients diagnosed with COVID-19 in the ambulatory setting, including patients diagnosed during the Delta-dominant period prior to Omicron emergence. Design Retrospective cohort study. Setting Komodo Health closed claims database. Participants 13 273 128 patients diagnosed with COVID-19 (December 2020 through September 2021) were treated with CAS+IMD or untreated but treatment eligible under the Emergency Use Authorization (EUA). Each treated patient was exact and propensity score matched without replacement to up to five untreated EUA-eligible patients.
Interventions CAS+IMD. Primary and secondary outcome measures Composite endpoint of 30-day all-cause mortality or COVID-19related hospitalisation. Kaplan-Meier estimators were used to calculate outcome risks overall and across subgroups: age, COVID-19 vaccination status, immunocompromised status, and timing of diagnosis (December 2020 to June 2021, and July to September 2021). Cox proportional hazards models were used to estimate adjusted HRs (aHRs) and 95% CIs. Results Among 75 159 CAS+IMD-treated and 1 670 338 EUA-eligible untreated patients, 73 759 treated patients were matched to 310 688 untreated patients; matched patients were ~50 years, ~60% were women and generally well balanced across risk factors. The 30-day risk of the composite outcome was 2.1% and 5.2% in the CAS+IMD-treated and CAS+IMD-untreated patients, respectively; equivalent to a 60% lower risk (aHR 0.40; 95% CI, 0.38 to 0.42). The effect of CAS+IMD was consistent across subgroups, including those who received a COVID-19 vaccine (aHR 0.48, 95% CI, 0.41 to 0.56), and those diagnosed during the Delta-dominant period (aHR 0.40, 95% CI, 0.38 to 0.42). Conclusions The real-world effectiveness of CAS+IMD is consistent with the efficacy for reducing all-cause mortality or COVID-19-related hospitalisation reported in clinical trials. Effectiveness is maintained across patient subgroups, including those prone to breakthrough infections, and was effective against susceptible variants including Delta.
Ethics approval This study was conducted as secondary research using deidentified data licensed from a third party, Komodo, in compliance with 45 CFR 164.514(a)-(c). The data had identifying patient information removed and were coded in such a way that they could not be linked back to subjects from whom they were originally collected prior to the authors gaining access. This research did not require institutional review board or ethics review, as analyses of these data do not meet the definition of 'research involving human subjects' as defined within 45 CFR 46.102(f), which stipulates human subjects as living individuals about whom an investigator obtains identifiable private information for research purposes. Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as online supplemental information. All data generated or analysed during this study are included in this published article. Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the..
References
Allen, Vusirikala, Flannagan, Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): National case-control study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2021.100252
Anderson, Smith, Edupuganti, Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19, Open Forum Infect Dis, doi:10.1016/j.jcv.2021.105026
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm Stat, doi:10.1002/pst.433
Bast, Tang, Dahn, Increased risk of hospitalisation and death with the delta variant in the USA, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00685-X
Bierle, Ganesh, Tulledge-Scheitel, Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities, J Infect Dis, doi:10.1093/infdis/jiab570
Bierle, Ganesh, Wilker, Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19, J Prim Care Community Health, doi:10.1177/21501327211019282
Boyapati, Wipperman, Ehmann, Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial, J Infect Dis, doi:10.1093/infdis/jiab445
Cavazzoni, Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant
Charlson, Pompei, Ales, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis, doi:10.1016/0021-9681(87)90171-8
Chilimuri, Mantri, Gurjar, Implementation and outcomes of monoclonal antibody infusion for COVID-19 in an inner-city safety net hospital: a South-Bronx experience, J Natl Med Assoc, doi:10.1016/j.jnma.2021.08.036
Christensen, Olsen, Long, Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas, Am J Pathol, doi:10.1016/j.ajpath.2021.10.019
Close, Jones, Jentoft, Outcome comparison of high-risk Native American patients who did or did not receive monoclonal antibody treatment for COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.25866
Cooper, Christensen, Salazar, Real-world assessment of 2879 COVID-19 patients treated with monoclonal antibody on December 20, Open Forum Infect Dis, doi:10.1093/ofid/ofab512
Copin, Baum, Wloga, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell, doi:10.1016/j.cell.2021.06.002
D'agostino, Lang, Walkup, Examining the impact of missing data on propensity score estimation in determining the effectiveness of self-monitoring of blood glucose (SMBG), Health Serv Outcomes Res Methodol, doi:10.1023/A:1020375413191
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Falcone, Tiseo, Valoriani, Efficacy of bamlanivimab/ etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern, Infect Dis Ther, doi:10.1007/s40121-021-00525-4
Fisman, Tuite, Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada, CMAJ, doi:10.1503/cmaj.211248
Ganesh, Philpot, Bierle, Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019, J Infect Dis, doi:10.1093/infdis/jiab377
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Groenwold, White, Donders, Missing covariate data in clinical research: when and when not to use the missingindicator method for analysis, CMAJ, doi:10.1503/cmaj.110977
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hall, Wellner, Confidence bands for a survival curve from censored data, Biometrika, doi:10.1093/biomet/67.1.133
Jones, Faruqi, Sullivan, COVID-19 outcomes in patients undergoing B cell depletion therapy and those with humoral immunodeficiency states: a scoping review, Pathog Immun, doi:10.20411/pai.v6i1.435
Kakinoki, Yamada, Tanino, Impact of antibody cocktail therapy combined with Casirivimab and Imdevimab on clinical outcome for patients with COVID-19 in a real-life setting: a single Institute analysis, Int J Infect Dis, doi:10.1016/j.ijid.2022.01.067
Kaplan, Meier, Nonparametric estimation from incomplete observations, J Am Stat Assoc, doi:10.1080/01621459.1958.10501452
Kislaya, Rodrigues, Borges, Comparative effectiveness of coronavirus vaccine in preventing breakthrough infections among vaccinated persons infected with delta and alpha variants, Emerg Infect Dis, doi:10.3201/eid2802.211789
Lin, Wei, The robust inference for the COX proportional hazards model, J Am Stat Assoc, doi:10.1080/01621459.1989.10478874
Luo, Morris, Sachithanandham, Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals, Clin Infect Dis, doi:10.1093/cid/ciab986
Magesh, John, Li, Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.34147
Marques, Sherrill-Mix, Everett, SARS-CoV-2 variants associated with vaccine breakthrough in the Delaware Valley through summer 2021, mBio, doi:10.1128/mbio.03788-21
Pham, Murugesan, Banaei, Immunogenicity and tolerability of COVID-19 messenger RNA vaccines in primary immunodeficiency patients with functional B-cell defects, J Allergy Clin Immunol, doi:10.1016/j.jaci.2021.11.022
Piccicacco, Zeitler, Montero, Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients, Open Forum Infect Dis, doi:10.1093/ofid/ofab292
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Planas, Veyer, Baidaliuk, Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Qu, Lipkovich, Propensity scoring with missing values
Razonable, Aloia, Anderson, A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate coronavirus Disease-19: the Mayo clinic model, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.03.010
Razonable, Pawlowski, Horo, Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101102
Shields, Burns, Savic, COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.12.620
Stuart, Lee, Leacy, Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research, J Clin Epidemiol, doi:10.1016/j.jclinepi.2013.01.013
Syed, Ciling, Taha, Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 viruslike particles, Proc Natl Acad Sci, doi:10.1073/pnas.2200592119
Trunfio, Venuti, Alladio, Diagnostic SARS-CoV-2 cycle threshold value predicts disease severity, survival, and six-month sequelae in COVID-19 symptomatic patients, Viruses, doi:10.3390/v13020281
Twohig, Nyberg, Zaidi, Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00475-8
Verderese, Stepanova, Lam, Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate coronavirus disease 2019 (COVID-19): a real-world experience, Clin Infect Dis, doi:10.1093/cid/ciab579
Wadman, What does the delta variant have in store for the United States? We asked coronavirus experts
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature, doi:10.1038/s41586-021-03398-2
Webb, Buckel, Vento, Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofab331
Wei, Murdock, Jalbert, Real-world effectiveness of Casirivimab and Imdevimab in patients with COVID-19 in the ambulatory setting: an analysis of two large US national claims databases, doi:10.1101/2022.02.28.22270796
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wilhelm, Toptan, Pallas, Antibody-mediated neutralization of authentic SARS-CoV-2 B.1.617 variants harboring L452R and T478K/E484Q, Viruses, doi:10.3390/v13091693
Wilhelm, Widera, Grikscheit, Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies, medRxiv, doi:10.1101/2021.12.07.21267432v2
DOI record:
{
"DOI": "10.1136/bmjopen-2022-064953",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2022-064953",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To assess the real-world effectiveness of casirivimab and imdevimab (CAS+IMD) versus no COVID-19 antibody treatment among patients diagnosed with COVID-19 in the ambulatory setting, including patients diagnosed during the Delta-dominant period prior to Omicron emergence.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Retrospective cohort study.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Komodo Health closed claims database.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>13 273 128 patients diagnosed with COVID-19 (December 2020 through September 2021) were treated with CAS+IMD or untreated but treatment eligible under the Emergency Use Authorization (EUA). Each treated patient was exact and propensity score matched without replacement to up to five untreated EUA-eligible patients.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>CAS+IMD.</jats:p></jats:sec><jats:sec><jats:title>Primary and secondary outcome measures</jats:title><jats:p>Composite endpoint of 30-day all-cause mortality or COVID-19-related hospitalisation. Kaplan-Meier estimators were used to calculate outcome risks overall and across subgroups: age, COVID-19 vaccination status, immunocompromised status, and timing of diagnosis (December 2020 to June 2021, and July to September 2021). Cox proportional hazards models were used to estimate adjusted HRs (aHRs) and 95% CIs.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 75 159 CAS+IMD-treated and 1 670 338 EUA-eligible untreated patients, 73 759 treated patients were matched to 310 688 untreated patients; matched patients were ~50 years, ~60% were women and generally well balanced across risk factors. The 30-day risk of the composite outcome was 2.1% and 5.2% in the CAS+IMD-treated and CAS+IMD-untreated patients, respectively; equivalent to a 60% lower risk (aHR 0.40; 95% CI, 0.38 to 0.42). The effect of CAS+IMD was consistent across subgroups, including those who received a COVID-19 vaccine (aHR 0.48, 95% CI, 0.41 to 0.56), and those diagnosed during the Delta-dominant period (aHR 0.40, 95% CI, 0.38 to 0.42).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>The real-world effectiveness of CAS+IMD is consistent with the efficacy for reducing all-cause mortality or COVID-19-related hospitalisation reported in clinical trials. Effectiveness is maintained across patient subgroups, including those prone to breakthrough infections, and was effective against susceptible variants including Delta. </jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2022-064953"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6138-7021",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hussein",
"given": "Mohamed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wei",
"given": "Wenhui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mastey",
"given": "Vera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanchez",
"given": "Robert J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Degang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murdock",
"given": "Dana J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirshberg",
"given": "Boaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weinreich",
"given": "David M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jalbert",
"given": "Jessica J",
"sequence": "additional"
}
],
"container-title": "BMJ Open",
"container-title-short": "BMJ Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T16:22:42Z",
"timestamp": 1671466962000
},
"deposited": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T16:23:05Z",
"timestamp": 1671466985000
},
"funder": [
{
"DOI": "10.13039/100009857",
"award": [
"NA"
],
"doi-asserted-by": "crossref",
"name": "Regeneron Pharmaceuticals, Inc"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T05:56:07Z",
"timestamp": 1671515767163
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2022,
12,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 18,
"start": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T00:00:00Z",
"timestamp": 1671408000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2022-064953",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e064953",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1056/nejmoa2035002",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.1"
},
{
"DOI": "10.1056/nejmoa2108163",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.2"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2022121908201429000_12.12.e064953.3",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "2022121908201429000_12.12.e064953.4",
"volume": "385",
"year": "2021"
},
{
"key": "2022121908201429000_12.12.e064953.5",
"unstructured": "U.S. Food & Drug Administration . Fact sheet for health care providers emergency use Authorization (EUA) of Casirivimab and Imdevimab, 2022. Available: https://www.fda.gov/media/145611/download [Accessed 06 Mar 2022]."
},
{
"key": "2022121908201429000_12.12.e064953.6",
"unstructured": "U.S. Food & Drug Administration . Fact sheet for health care providers. emergency use Authorization (EUA) of Bamlanivimab and Etesevimab, 2021. Available: https://www.fda.gov/media/145802/download [Accessed 21 Jan 2022]."
},
{
"key": "2022121908201429000_12.12.e064953.7",
"unstructured": "U.S. Food & Drug Administration . Fact sheet for health care providers. emergency use Authorization (EUA) of Sotrovimab, 2021. Available: https://www.fda.gov/media/149534/download [Accessed 21 Jan 2022]."
},
{
"DOI": "10.1016/j.lanepe.2021.100252",
"article-title": "Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): National case-control study",
"author": "Allen",
"doi-asserted-by": "crossref",
"first-page": "100252",
"journal-title": "Lancet Reg Health Eur",
"key": "2022121908201429000_12.12.e064953.8",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1101/2021.08.15.21262077",
"doi-asserted-by": "crossref",
"key": "2022121908201429000_12.12.e064953.9",
"unstructured": "Luo CH , Morris CP , Sachithanandham J , et al . Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin Infect Dis 2021.doi:10.1093/cid/ciab986"
},
{
"DOI": "10.1016/S1473-3099(21)00685-X",
"article-title": "Increased risk of hospitalisation and death with the delta variant in the USA",
"author": "Bast",
"doi-asserted-by": "crossref",
"first-page": "1629",
"journal-title": "Lancet Infect Dis",
"key": "2022121908201429000_12.12.e064953.10",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1503/cmaj.211248",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.11"
},
{
"DOI": "10.1016/s1473-3099(21)00475-8",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.12"
},
{
"DOI": "10.1056/NEJMc2031364",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.13"
},
{
"DOI": "10.1016/j.cell.2021.06.002",
"article-title": "The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies",
"author": "Copin",
"doi-asserted-by": "crossref",
"first-page": "3949",
"journal-title": "Cell",
"key": "2022121908201429000_12.12.e064953.14",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.3390/v13091693",
"article-title": "Antibody-mediated neutralization of authentic SARS-CoV-2 B.1.617 variants harboring L452R and T478K/E484Q",
"author": "Wilhelm",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "2022121908201429000_12.12.e064953.15",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1101/2021.05.26.445838",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.16"
},
{
"DOI": "10.1073/pnas.2200592119",
"doi-asserted-by": "crossref",
"key": "2022121908201429000_12.12.e064953.17",
"unstructured": "Syed AM , Ciling A , Taha TY , et al . Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci U S A 2022;119.doi:10.1073/pnas.2200592119"
},
{
"DOI": "10.1093/ofid/ofab512",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.18"
},
{
"DOI": "10.1093/infdis/jiab570",
"article-title": "Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities",
"author": "Bierle",
"doi-asserted-by": "crossref",
"first-page": "598",
"journal-title": "J Infect Dis",
"key": "2022121908201429000_12.12.e064953.19",
"volume": "225",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiab377",
"article-title": "Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019",
"author": "Ganesh",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "J Infect Dis",
"key": "2022121908201429000_12.12.e064953.20",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocp.2021.03.010",
"article-title": "A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate coronavirus Disease-19: the Mayo clinic model",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "1250",
"journal-title": "Mayo Clin Proc",
"key": "2022121908201429000_12.12.e064953.21",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab579",
"article-title": "Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate coronavirus disease 2019 (COVID-19): a real-world experience",
"author": "Verderese",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Clin Infect Dis",
"key": "2022121908201429000_12.12.e064953.22",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofab331",
"article-title": "Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19",
"author": "Webb",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2022121908201429000_12.12.e064953.23",
"volume": "8",
"year": "2021"
},
{
"article-title": "Implementation and outcomes of monoclonal antibody infusion for COVID-19 in an inner-city safety net hospital: a South-Bronx experience",
"author": "Chilimuri",
"first-page": "701",
"journal-title": "J Natl Med Assoc",
"key": "2022121908201429000_12.12.e064953.24",
"volume": "113",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.25866",
"article-title": "Outcome comparison of high-risk Native American patients who did or did not receive monoclonal antibody treatment for COVID-19",
"author": "Close",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "2022121908201429000_12.12.e064953.25",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1101/2021.10.10.21264589",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.26"
},
{
"DOI": "10.1093/ofid/ofab292",
"article-title": "Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2022121908201429000_12.12.e064953.27",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00525-4",
"article-title": "Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern",
"author": "Falcone",
"doi-asserted-by": "crossref",
"first-page": "2479",
"journal-title": "Infect Dis Ther",
"key": "2022121908201429000_12.12.e064953.28",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab315",
"article-title": "Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019",
"author": "Anderson",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2022121908201429000_12.12.e064953.29",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1101/2021.11.30.21266756",
"doi-asserted-by": "crossref",
"key": "2022121908201429000_12.12.e064953.30",
"unstructured": "McCreary EK , Bariola JR , Wadas RJ , et al . Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19. medRxiv 2021.doi:10.1101/2021.11.30.21266756"
},
{
"DOI": "10.1016/j.jcv.2021.105026",
"article-title": "Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge",
"author": "Bierle",
"doi-asserted-by": "crossref",
"first-page": "105026",
"journal-title": "J Clin Virol",
"key": "2022121908201429000_12.12.e064953.31",
"volume": "145",
"year": "2021"
},
{
"key": "2022121908201429000_12.12.e064953.32",
"unstructured": "Komodo Health Inc . Komodo health, 2021. Available: https://www.komodohealth.com/ [Accessed 23 Nov 2021]."
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.33"
},
{
"key": "2022121908201429000_12.12.e064953.34",
"unstructured": "Centers for Disease Control and Prevention . Clinical growth charts, 2017. Available: https://www.cdc.gov/growthcharts/clinical_charts.htm [Accessed 23 Nov 2021]."
},
{
"key": "2022121908201429000_12.12.e064953.35",
"unstructured": "Wadman M . What does the delta variant have in store for the United States? We asked coronavirus experts, 2021. Available: https://www.science.org/content/article/what-does-delta-variant-have-store-us-we-asked-coronavirus-experts [Accessed 06 Mar 2022]."
},
{
"DOI": "10.1002/pst.433",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.36"
},
{
"DOI": "10.1023/A:1020375413191",
"article-title": "Examining the impact of missing data on propensity score estimation in determining the effectiveness of self-monitoring of blood glucose (SMBG)",
"author": "D'Agostino Jr.",
"doi-asserted-by": "crossref",
"first-page": "291",
"journal-title": "Health Serv Outcomes Res Methodol",
"key": "2022121908201429000_12.12.e064953.37",
"volume": "2",
"year": "2001"
},
{
"DOI": "10.1503/cmaj.110977",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.38"
},
{
"key": "2022121908201429000_12.12.e064953.39",
"unstructured": "Qu Y , Lipkovich I . Propensity scoring with missing values. In: Faries DE , Leon AC , Haro JM , et al , eds. Analysis of observational health care data using SAS Cary. Josep Maria Haro: SAS Publishing, 2010: 105–28."
},
{
"DOI": "10.1016/j.jclinepi.2013.01.013",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.40"
},
{
"DOI": "10.2307/2281868",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.41"
},
{
"DOI": "10.1093/biomet/67.1.133",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.42"
},
{
"DOI": "10.2307/2290085",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.43"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "101102",
"journal-title": "EClinicalMedicine",
"key": "2022121908201429000_12.12.e064953.44",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1101/2022.02.28.22270796",
"doi-asserted-by": "crossref",
"key": "2022121908201429000_12.12.e064953.45",
"unstructured": "Wei W , Murdock D , Jalbert JJ , et al . Real-world effectiveness of Casirivimab and Imdevimab in patients with COVID-19 in the ambulatory setting: an analysis of two large US national claims databases. medRxiv 2022.doi:10.1101/2022.02.28.22270796"
},
{
"DOI": "10.3201/eid2802.211789",
"article-title": "Comparative effectiveness of coronavirus vaccine in preventing breakthrough infections among vaccinated persons infected with delta and alpha variants",
"author": "Kislaya",
"doi-asserted-by": "crossref",
"first-page": "331",
"journal-title": "Emerg Infect Dis",
"key": "2022121908201429000_12.12.e064953.46",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2021.10.019",
"article-title": "Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas",
"author": "Christensen",
"doi-asserted-by": "crossref",
"first-page": "320",
"journal-title": "Am J Pathol",
"key": "2022121908201429000_12.12.e064953.47",
"volume": "192",
"year": "2022"
},
{
"DOI": "10.1128/mbio.03788-21",
"article-title": "SARS-CoV-2 variants associated with vaccine breakthrough in the Delaware Valley through summer 2021",
"author": "Marques",
"doi-asserted-by": "crossref",
"journal-title": "mBio",
"key": "2022121908201429000_12.12.e064953.48",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.jaci.2020.12.620",
"article-title": "COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience",
"author": "Shields",
"doi-asserted-by": "crossref",
"first-page": "870",
"journal-title": "J Allergy Clin Immunol",
"key": "2022121908201429000_12.12.e064953.49",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.20411/pai.v6i1.435",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.50"
},
{
"DOI": "10.1016/j.jaci.2021.11.022",
"article-title": "Immunogenicity and tolerability of COVID-19 messenger RNA vaccines in primary immunodeficiency patients with functional B-cell defects",
"author": "Pham",
"doi-asserted-by": "crossref",
"first-page": "907",
"journal-title": "J Allergy Clin Immunol",
"key": "2022121908201429000_12.12.e064953.51",
"volume": "149",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.52"
},
{
"DOI": "10.3390/v13020281",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.53"
},
{
"DOI": "10.1093/infdis/jiab445",
"doi-asserted-by": "publisher",
"key": "2022121908201429000_12.12.e064953.54"
},
{
"DOI": "10.1177/21501327211019282",
"article-title": "Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19",
"author": "Bierle",
"doi-asserted-by": "crossref",
"journal-title": "J Prim Care Community Health",
"key": "2022121908201429000_12.12.e064953.55",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.34147",
"article-title": "Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis",
"author": "Magesh",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "2022121908201429000_12.12.e064953.56",
"volume": "4",
"year": "2021"
},
{
"key": "2022121908201429000_12.12.e064953.57",
"unstructured": "Cavazzoni P . Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. Available: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron [Accessed 01 Feb 2022]."
},
{
"DOI": "10.1101/2021.12.07.21267432",
"doi-asserted-by": "crossref",
"key": "2022121908201429000_12.12.e064953.58",
"unstructured": "Wilhelm A , Widera M , Grikscheit K , et al . Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv 2021.doi:10.1101/2021.12.07.21267432v2"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022121908201429000_12.12.e064953.59",
"volume": "602",
"year": "2022"
}
],
"reference-count": 59,
"references-count": 59,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2022-064953"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "12"
}
