Real-world Assessment of 2,879 COVID-19 Patients Treated with Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab512, Oct 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
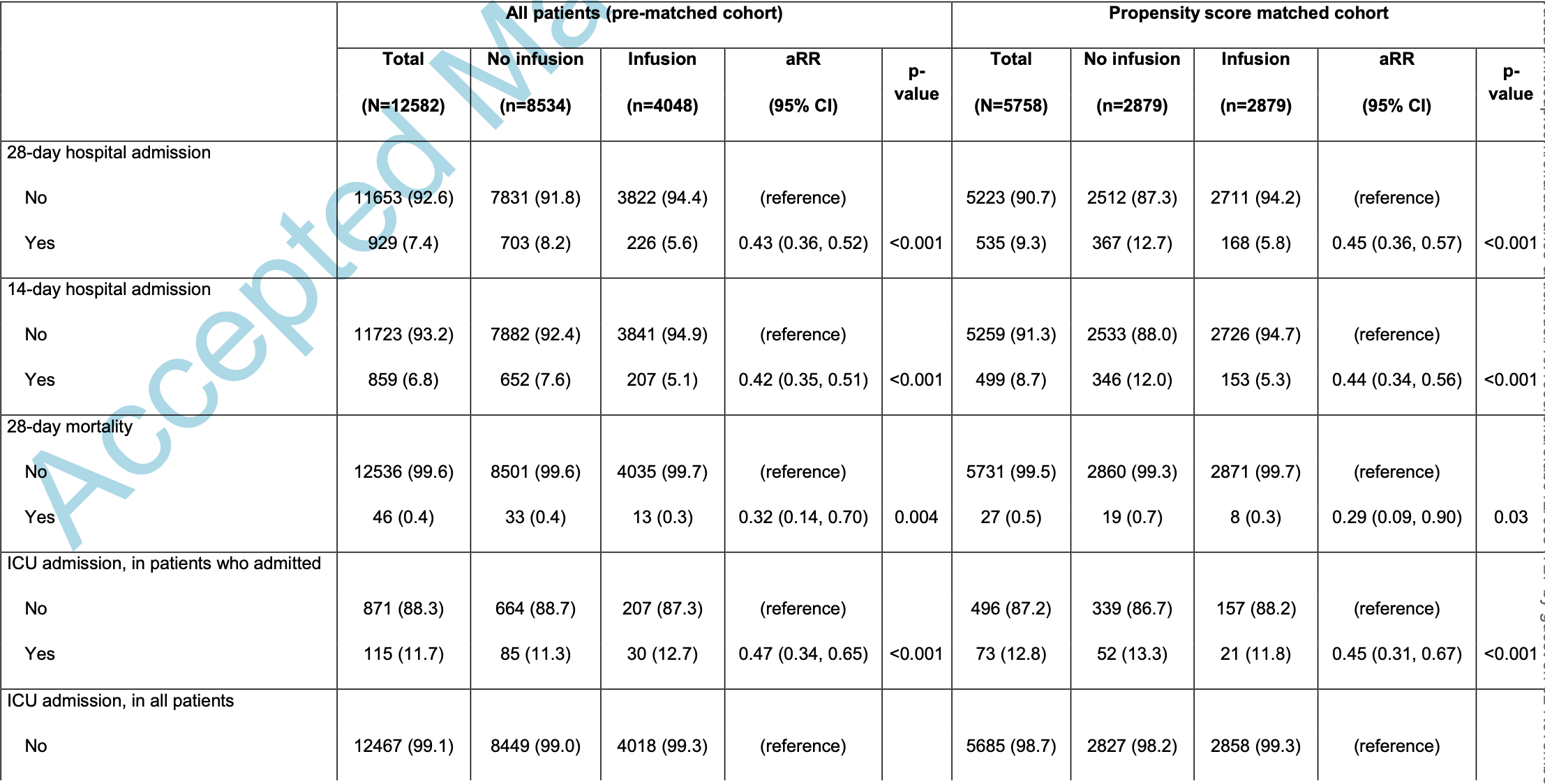
Retrospective 2,879 patients and matched controls in the USA, showing significantly lower mortality and hospitalization with bamlanivimab, bamlanivimab/etesevimab, and casirivimab/imdevimab. There was significantly lower hospitalization with casirivimab/imdevimab compared to bamlanivimab or bamlanivimab/etesevimab. PSM and multivariate analysis is only provided for all treatments combined.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details.
Study covers casirivimab/imdevimab and bamlanivimab/etesevimab.
|
risk of death, 77.5% lower, RR 0.23, p = 0.18, treatment 1 of 1,148 (0.1%), control 33 of 8,534 (0.4%), NNT 334, unadjusted.
|
|
risk of ICU admission, 47.5% lower, RR 0.52, p = 0.14, treatment 6 of 1,148 (0.5%), control 85 of 8,534 (1.0%), NNT 211, unadjusted.
|
|
risk of hospitalization, 52.4% lower, RR 0.48, p < 0.001, treatment 45 of 1,148 (3.9%), control 703 of 8,534 (8.2%), NNT 23, unadjusted.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Cooper et al., 8 Oct 2021, retrospective, USA, peer-reviewed, 9 authors.
Real-world Assessment of 2,879 COVID-19 Patients Treated with Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study
doi:10.1093/ofid/ofab512/6384727
We assessed the outcomes of 2,879 SARS-CoV-2-positive patients transfused with one of three available monoclonal antibody (mAbs) treatments compared to a propensity-matched control cohort. We found that administration of mAbs significantly reduced 28-day hospitalizations and mortality.
A c c e p t e d M a n u s c r i p t 14 Monoclonal antibodies are susceptible to the evolution of viral resistance mutations in target epitopes, and the EUA for bamlanivimab was revoked due to demonstrated resistance of emerging variants (https://www.fda.gov/media/147629/download). Similarly, the EUA for bamlanivimab and etesevimab was recently revoked (June 25, 2021), due to the increasing prevalence of the P.1 and B.1.351 variants (www.covid19.lilly.com/bam-ete) that have been shown to escape certain mAbs. 23, 26 Combination mAbs that bind non-overlapping epitopes reduce the risk of resistance and have a clear advantage, and continued monitoring and assessment of emerging variants and their susceptibility to natural or induced immunity and therapeutic antibodies remains crucial. In summary, our propensity score-matched analysis confirms that early infusion of neutralizing mAbs to COVID-19 patients significantly reduced hospitalization and mortality among a large cohort of high-risk individuals. These data support continued widespread efforts to make early passive immune therapy available to COVID-19 patients.
Potential Conflicts of Interest All authors report no conflicts of interest related to this study.
A c c e p t e d M a n u s c r i p t 15 The study was reviewed and approved by the Houston Methodist Research Institute Institutional Review Board (IRB PRO00029666). Given the study design, patient's written consent was not required. However, verbal and written..
References
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med
Berry, Liebl, Todd, Brownewell, Rapid Operationalization of Covid-19 Monoclonal Antibody Infusion Clinics, NEJM Catalyst
Brown, Mccullough, Treatment for emerging viruses: Convalescent plasma and COVID-19, Transfus Apher Sci
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med
Chen, Xiong, Bao, Shi, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect Dis
Dougan, Nirula, Azizad, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Duan, Liu, Li, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A
Fine, Gray, A Proportional Hazards Model for the Subdistribution of a Competing Risk, Journal of the American Statistical Association
Ganesh, Pawlowski, Horo, Association of Intravenous Bamlanivimab Use with Reduced Hospitalization, Intensive Care Unit Admission, and Mortality in Patients with Mild to Moderate COVID-19, medRxiv
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Hoffmann, Arora, Gross, SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies, Cell
Jenks, Aslam, Horton, Early Monoclonal Antibody Administration Can Reduce Both Hospitalizations and Mortality in High-Risk Outpatients with COVID-19, Clin Infect Dis
Kumar, Wu, Stosor, Real-World Experience of Bamlanivimab for COVID-19: A Case-Control Study, Clin Infect Dis
Long, Olsen, Christensen, Molecular Architecture of Early Dissemination and Massive Second Wave of the SARS-CoV-2 Virus in a Major Metropolitan Area, mBio
Long, Olsen, Christensen, Sequence Analysis of 20,453 Severe Acute Respiratory Syndrome Coronavirus 2 Genomes from the Houston Metropolitan Area
Salazar, Christensen, Graviss, Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG, Am J Pathol
Salazar, Perez, Ashraf, Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma, Am J Pathol
Stuart, Lee, Leacy, Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research, J Clin Epidemiol
Verity, Okell, Dorigatti, Estimates of the severity of coronavirus disease 2019: a model-based analysis, Lancet Infect Dis
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Cocktail in Outpatients with Covid-19, medRxiv
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Widera, Wilhelm, Hoehl, Limited neutralization of authentic SARS-CoV-2 variants carrying E484K in vitro, J Infect Dis
DOI record:
{
"DOI": "10.1093/ofid/ofab512",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofab512",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>SARS-CoV-2 continues to spread globally and cause significant morbidity and mortality. Anti-spike protein monoclonal antibody (mAb) therapy has been shown to prevent progression to severe COVID-19 disease. The objective of this study was to report the outcomes of high-risk, SARS-CoV-2-positive patients infused with one of the three mAb available through FDA emergency use authorization (EUA).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A total of 4,328 SARS-CoV-2-positive patients that satisfied EUA criteria for eligibility for receiving mAb therapy were infused with bamlanivimab or combination therapies bamlanivimab-etesevimab or casirivimab-imdevimab from November 22, 2020, to May 31, 2021, at six infusion clinics and multiple emergency departments within the eight Houston Methodist Hospitals in Houston, Texas. The primary outcome of hospital admission within 14- and 28-days post-infusion was assessed relative to a propensity-score matched cohort, matched based on age, race/ethnicity, median income by zip code, body mass index, comorbidities, and positive PCR date. Secondary outcomes included ICU admission and mortality.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 2,879 infused patients and matched controls were included in the analysis, including 1,718 patients infused with bamlanivimab, 346 patients infused with bamlanivimab-etesevimab, and 815 patients infused with casirivimab-imdevimab. Hospital admission and mortality rates were significantly decreased overall in mAb-infused patients relative to matched controls. Among the infused cohort, those who received casirivimab-imdevimab had significantly decreased rate of admission relative to the other two mAbs groups (aRR = 0.51, p=0.001).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Treatment with bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab significantly decreased the number of patients who progressed to severe COVID-19 disease and required hospitalization.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7531-4376",
"affiliation": [
{
"name": "Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA"
}
],
"authenticated-orcid": false,
"family": "Cooper",
"given": "Megan H",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, Texas, USA"
}
],
"family": "Christensen",
"given": "Paul A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, Texas, USA"
}
],
"family": "Salazar",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA"
},
{
"name": "Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, Texas, USA"
}
],
"family": "Perez",
"given": "Katherine K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Molecular and Translational Human Infectious Diseases Research, Houston Methodist Research Institute, Houston, Texas, USA"
}
],
"family": "Graviss",
"given": "Edward A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Molecular and Translational Human Infectious Diseases Research, Houston Methodist Research Institute, Houston, Texas, USA"
}
],
"family": "Nguyen",
"given": "Duc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, Texas, USA"
},
{
"name": "Center for Molecular and Translational Human Infectious Diseases Research, Houston Methodist Research Institute, Houston, Texas, USA"
}
],
"family": "Musser",
"given": "James M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonology, Pulmonary, Critical Care & Sleep Medicine, Houston Methodist Hospital, Houston, TX, USA"
}
],
"family": "Huang",
"given": "Howard J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA"
}
],
"family": "Liebl",
"given": "Michael G",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
9
]
],
"date-time": "2021-10-09T01:52:08Z",
"timestamp": 1633744328000
},
"deposited": {
"date-parts": [
[
2021,
10,
9
]
],
"date-time": "2021-10-09T01:52:10Z",
"timestamp": 1633744330000
},
"indexed": {
"date-parts": [
[
2023,
5,
8
]
],
"date-time": "2023-05-08T07:52:32Z",
"timestamp": 1683532352537
},
"is-referenced-by-count": 9,
"issued": {
"date-parts": [
[
2021,
10,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
8
]
],
"date-time": "2021-10-08T00:00:00Z",
"timestamp": 1633651200000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofab512/40533751/ofab512.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofab512/40533751/ofab512.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
10,
8
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
8
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofab512/6384727"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Real-world Assessment of 2,879 COVID-19 Patients Treated with Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study",
"type": "journal-article"
}
cooper
