Efficacy of N‑Acetylcysteine in Children with Moderate COVID-19: A Placebo-Controlled Randomized Clinical Trial
et al., Journal of Comprehensive Pediatrics, doi:10.5812/jcp-139612, IRCT20220516054879N1, Apr 2024
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
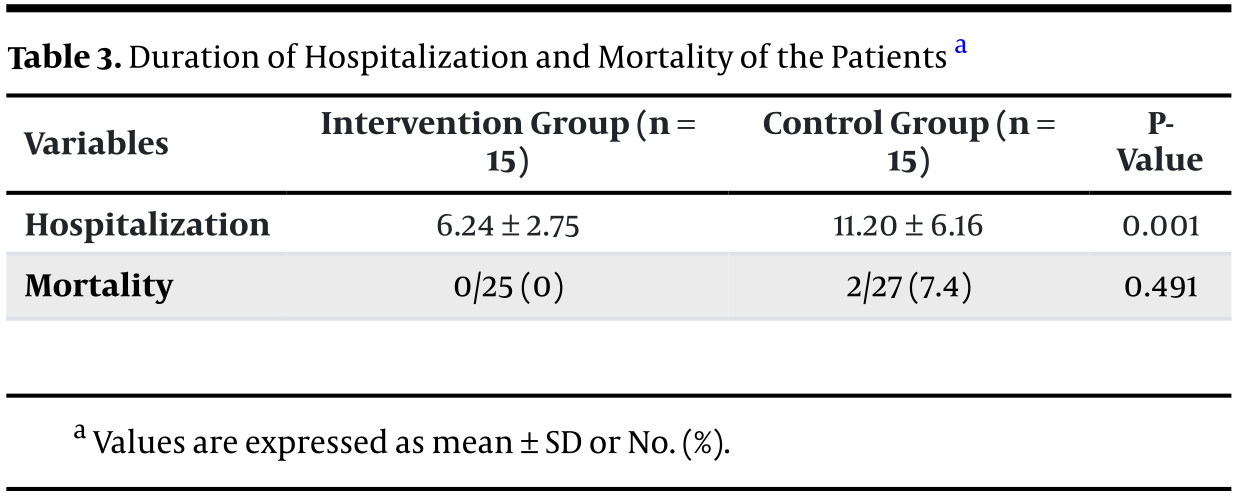
RCT 58 hospitalized children with moderate COVID-19 showing shorter hospitalization and improved oxygen saturation with N-acetylcysteine treatment. However, baseline oxygen values are inconsistent with the reported inclusion criteria - the inclusion criteria show SpO2 ≥ 94%, however baseline means are 86 and 89. The day 7 treatment WBC median is larger than the Q3 values. The Table 3 header shows only 15 patients per group without explanation.
This study is excluded in meta-analysis:
potential data issue.
|
risk of death, 79.4% lower, RR 0.21, p = 0.49, treatment 0 of 25 (0.0%), control 2 of 27 (7.4%), NNT 14, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
hospitalization time, 44.3% lower, relative time 0.56, p = 0.008, treatment mean 6.24 (±2.75) n=15, control mean 11.2 (±6.16) n=15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hashemian et al., 6 Apr 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 9 authors, study period June 2022 - December 2022, trial IRCT20220516054879N1.
Contact: shahrokhi.mail85@gmail.com.
Efficacy of N‑Acetylcysteine in Children with Moderate COVID-19: A Placebo-Controlled Randomized Clinical Trial
Journal of Comprehensive Pediatrics, doi:10.5812/jcp-139612
Background: The full scope of coronavirus disease 2019 (COVID-19) remains unknown, and a definitive treatment for children has yet to be established. N-acetylcysteine (NAC), beyond its mucolytic effect in lung disorders, operates through various mechanisms, such as enhancing the immune system, inhibiting viral replication, and reducing inflammation. These pharmacological properties of NAC suggest it is a potential therapeutic agent for COVID-19. Objectives: Our goal was to evaluate whether NAC could improve outcomes in hospitalized children presenting with acute respiratory symptoms due to COVID-19. Methods: Fifty-eight patients with moderate COVID-19 symptoms were randomly allocated to receive either 1200 mg/day of NAC or a placebo for 7 days. We monitored NAC-related side effects, C-reactive protein (CRP) levels, white blood cell (WBC) count, serum creatinine, oxygen saturation, hospital stay duration, and clinical symptoms. Results: All measured variables in both groups showed significant improvement by the end of the study. However, the analysis indicated that the changes in CRP and WBC levels in the NAC group, compared to the placebo, were not significant (P = 0.659 and 0.067, respectively). There was a notable improvement in oxygen saturation in the NAC group versus the placebo group at the study's conclusion (P = 0.001). The length of hospital stay and CRP levels significantly decreased in the NAC group compared to the placebo group (P-value = 0.001 and P-value ≤ 0.001, respectively). Additionally, the mortality rate was 0.0% in the intervention group versus 7.4% in the placebo group (P-value = 0.491). Conclusions: The findings from this study support the potential of NAC in shortening hospital stay durations and enhancing oxygen saturation among children with COVID-19.
Funding/Support: Maryam Shahrokhi received the funding for this research project from the Guilan University of Medical Sciences funding source (Grant number: 3707-3533). Informed Consent: Written informed consent was obtained from the parents or guardians of the patients.
References
Assimakopoulos, Aretha, Komninos, Dimitropoulou, Lagadinou et al., N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study, Infect Dis (Lond), doi:10.1080/23744235.2021.1945675
Baud, Qi, Nielsen-Saines, Musso, Pomar et al., Real estimates of mortality following COVID-19 infection, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30195-X
Bhattacharya, Mondal, Naiya, Lyngdoh, Mukherjee et al., The beneficial role of N-acetylcysteine as an adjunctive drug in treatment of COVID-19 patients in a tertiary care hospital in India: an observational study, Int J Res Med Sci
Cazzola, Calzetta, Page, Rogliani, Matera, Thiol-Based Drugs in Pulmonary Medicine: Much More than Mucolytics, Trends Pharmacol Sci, doi:10.1016/j.tips.2019.04.015
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Dong, Mo, Hu, Qi, Jiang et al., Epidemiology of COVID-19 Among Children in China, Pediatr, doi:10.1542/peds.2020-0702
Ehre, Rushton, Wang, Hothem, Morrison et al., An Improved Inhaled Mucolytic to Treat Airway Muco-obstructive Diseases, Am J Respir Crit Care Med, doi:10.1164/rccm.201802-0245OC
Ershad, Naji, Vearrier, N-Acetylcysteine. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Abdullah Naji declares no relevant financial relationships with ineligible companies. Disclosure: David Vearrier declares no relevant financial relationships with ineligible companies
Fisher, Curry, Evaluation and treatment of acetaminophen toxicity, Adv Pharmacol, doi:10.1016/bs.apha.2018.12.004
Hashemipour, COVID-19 and Diabetes in Children: A Narrative Review, J Pediatr Rev, doi:10.32598/jpr.10.SpecialIssue.584.3
Hoffmann, Fischereder, Kruger, Drobnik, Kramer, The value of N-acetylcysteine in the prevention of radiocontrast agentinduced nephropathy seems questionable, J Am Soc Nephrol, doi:10.1097/01.asn.0000106780.14856.55
Huang, Clarkin, Mccudden, Akbari, Chow et al., The Effect of N-Acetylcysteine on Creatinine Measurement: Protocol for a Systematic Review, Can J Kidney Health Dis, doi:10.1177/2054358118801017
Ibrahim, Smith, Lewis, Kon, Goldenberg, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol, doi:10.1016/j.clim.2020.108544
Ladhani, Amin-Chowdhury, Davies, Aiano, Hayden et al., COVID-19 in children: analysis of the first pandemic peak in England, Arch Dis Child, doi:10.1136/archdischild-2020-320042
Lee, Hynan, Rossaro, Fontana, Stravitz et al., Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure, Gastroenterol, doi:10.1053/j.gastro.2009.06.006
Li, Welling, Johnson, Coughlin, Mulqueen et al., N-Acetylcysteine for Pediatric Obsessive-Compulsive Disorder: A Small Pilot Study, J Child Adolesc Psychopharmacol, doi:10.1089/cap.2019.0041
Liu, Liu, Xu, Xu, Huang et al., Ventilatory Ratio in Hypercapnic Mechanically Ventilated Patients with COVID-19associated Acute Respiratory Distress Syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.202002-0373LE
Liu, Smyth, Li, Qaseem, Florez et al., Guidelines for the prevention and management of children and adolescents with COVID-19, Eur J Pediatr, doi:10.1007/s00431-022-04615-4
Mohanty, Padhy, Das, Meher, Therapeutic potential of Nacetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence, Europ Rev Med Pharmacol Sci
Mp, N-acetylcysteine as a potential treatment for COVID-19, Future Microbiol, doi:10.2217/fmb-2020-0074
Panahi, Ghanei, Rahimi, Samim, Vahedian-Azimi et al., Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: An open-label randomized controlled clinical trial, J Med Virol, doi:10.1002/jmv.28393
Parri, Lenge, Buonsenso, Children with Covid-19 in Pediatric Emergency Departments in Italy, N Engl J Med, doi:10.1056/NEJMc2007617
Pasini, Stranieri, Cominacini, Mozzini, Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-Cov-2 Complications, Antioxid, doi:10.3390/antiox10020272
Rahimi, Samimagham, Azad, Hooshyar, Arabi et al., The efficacy of N-Acetylcysteine in severe COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-021-05242-4
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Shi, Puyo, N-Acetylcysteine to Combat COVID-19: An Evidence Review, Ther Clin Risk Manag, doi:10.2147/TCRM.S273700
Taher, Lashgari, Sedighi, Rahimi-Bashar, Poorolajal et al., A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome, Pharmacol Rep, doi:10.1007/s43440-021-00296-2
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Yang, Liu, Li, Zhao, Corona Virus Disease 2019, a growing threat to children?, J Infect, doi:10.1016/j.jinf.2020.02.024
Zhan, Doerfler, Xie, Chen, Chen et al., Association of Opioids and Nonsteroidal Anti-inflammatory Drugs With Outcomes in CKD: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study, Am J Kidney Dis, doi:10.1053/j.ajkd.2019.12.010
Zhou, Yang, Huang, Chen, The Potential Mechanism of Nacetylcysteine in Treating COVID-19, Curr Pharm Biotechnol, doi:10.2174/1389201021999201228212043
DOI record:
{
"DOI": "10.5812/jcp-139612",
"ISSN": [
"2251-8150",
"2251-8177"
],
"URL": "http://dx.doi.org/10.5812/jcp-139612",
"abstract": "<jats:p>Background: The full scope of coronavirus disease 2019 (COVID-19) remains unknown, and a definitive treatment for children has yet to be established. N-acetylcysteine (NAC), beyond its mucolytic effect in lung disorders, operates through various mechanisms, such as enhancing the immune system, inhibiting viral replication, and reducing inflammation. These pharmacological properties of NAC suggest it is a potential therapeutic agent for COVID-19. Objectives: Our goal was to evaluate whether NAC could improve outcomes in hospitalized children presenting with acute respiratory symptoms due to COVID-19. Methods: Fifty-eight patients with moderate COVID-19 symptoms were randomly allocated to receive either 1200 mg/day of NAC or a placebo for 7 days. We monitored NAC-related side effects, C-reactive protein (CRP) levels, white blood cell (WBC) count, serum creatinine, oxygen saturation, hospital stay duration, and clinical symptoms. Results: All measured variables in both groups showed significant improvement by the end of the study. However, the analysis indicated that the changes in CRP and WBC levels in the NAC group, compared to the placebo, were not significant (P = 0.659 and 0.067, respectively). There was a notable improvement in oxygen saturation in the NAC group versus the placebo group at the study's conclusion (P = 0.001). The length of hospital stay and CRP levels significantly decreased in the NAC group compared to the placebo group (P-value = 0.001 and P-value ≤ 0.001, respectively). Additionally, the mortality rate was 0.0% in the intervention group versus 7.4% in the placebo group (P-value = 0.491). Conclusions: The findings from this study support the potential of NAC in shortening hospital stay durations and enhancing oxygen saturation among children with COVID-19.</jats:p>",
"alternative-id": [
"8b3043a7cd16b39419753409a66bb4f777e39bdc"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2023-8-1"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2024-2-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-3-3"
},
{
"group": {
"label": "Import History",
"name": "import_history"
},
"label": "Import",
"name": "import",
"value": "Article is imported on 2024-04-06 13:00:42 by user ID: 131435."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0003-2927-899X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hashemian",
"given": "Houman",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-7587-2127",
"affiliation": [],
"authenticated-orcid": false,
"family": "Qobadighadikolaei",
"given": "Roja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seifnezhad",
"given": "Pouria",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6980-8866",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hassanzadeh Rad",
"given": "Afagh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sadat Mansouri",
"given": "Saeid",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0449-4605",
"affiliation": [],
"authenticated-orcid": false,
"family": "Darini",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jamali",
"given": "Faezeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rashidpour",
"given": "Fatemeh",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6162-750X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shahrokhi",
"given": "Maryam",
"sequence": "additional"
}
],
"container-title": "Journal of Comprehensive Pediatrics",
"container-title-short": "J Compr Ped",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"brieflands.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
6
]
],
"date-time": "2024-04-06T13:00:55Z",
"timestamp": 1712408455000
},
"deposited": {
"date-parts": [
[
2024,
11,
5
]
],
"date-time": "2024-11-05T21:55:47Z",
"timestamp": 1730843747000
},
"indexed": {
"date-parts": [
[
2025,
5,
14
]
],
"date-time": "2025-05-14T13:51:40Z",
"timestamp": 1747230700219,
"version": "3.40.5"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
4,
6
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
4,
6
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
3
]
],
"date-time": "2024-03-03T00:00:00Z",
"timestamp": 1709424000000
}
}
],
"link": [
{
"URL": "https://brieflands.com/articles/jcp-139612",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://brieflands.com/articles/jcp-139612",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3819",
"original-title": [],
"prefix": "10.5812",
"published": {
"date-parts": [
[
2024,
4,
6
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
6
]
]
},
"publisher": "Brieflands",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "key-A139612REF1-1"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "publisher",
"key": "key-A139612REF2-2"
},
{
"DOI": "10.1016/S1473-3099(20)30195-X",
"doi-asserted-by": "publisher",
"key": "key-A139612REF3-3"
},
{
"DOI": "10.32598/jpr.10.SpecialIssue.584.3",
"doi-asserted-by": "publisher",
"key": "key-A139612REF4-4"
},
{
"DOI": "10.1136/archdischild-2020-320042",
"doi-asserted-by": "publisher",
"key": "key-A139612REF5-5"
},
{
"DOI": "10.1056/NEJMc2007617",
"doi-asserted-by": "publisher",
"key": "key-A139612REF6-6"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "key-A139612REF7-7"
},
{
"author": "American Academy of Pediatrics ",
"journal-title": "Children and COVID-19: state-level data report.",
"key": "key-A139612REF8-8",
"year": "2021"
},
{
"DOI": "10.1542/peds.2020-0702",
"doi-asserted-by": "publisher",
"key": "key-A139612REF9-9"
},
{
"DOI": "10.1016/j.jinf.2020.02.024",
"doi-asserted-by": "publisher",
"key": "key-A139612REF10-10"
},
{
"DOI": "10.1007/s00431-022-04615-4",
"doi-asserted-by": "publisher",
"key": "key-A139612REF11-11"
},
{
"DOI": "10.1164/rccm.201802-0245OC",
"doi-asserted-by": "publisher",
"key": "key-A139612REF12-12"
},
{
"DOI": "10.1053/j.gastro.2009.06.006",
"doi-asserted-by": "publisher",
"key": "key-A139612REF13-13"
},
{
"DOI": "10.1016/bs.apha.2018.12.004",
"doi-asserted-by": "publisher",
"key": "key-A139612REF14-14"
},
{
"DOI": "10.1089/cap.2019.0041",
"doi-asserted-by": "publisher",
"key": "key-A139612REF15-15"
},
{
"DOI": "10.1016/j.tips.2019.04.015",
"doi-asserted-by": "publisher",
"key": "key-A139612REF16-16"
},
{
"DOI": "10.2147/TCRM.S273700",
"doi-asserted-by": "publisher",
"key": "key-A139612REF17-17"
},
{
"author": "Ershad M",
"journal-title": "StatPearls.",
"key": "key-A139612REF18-18",
"year": "2024"
},
{
"author": "National Institutes of Health ",
"journal-title": "COVID-19 Treatment Guidelines.",
"key": "key-A139612REF19-19",
"year": "2024"
},
{
"DOI": "10.1164/rccm.202002-0373LE",
"doi-asserted-by": "publisher",
"key": "key-A139612REF20-20"
},
{
"DOI": "10.1053/j.ajkd.2019.12.010",
"doi-asserted-by": "publisher",
"key": "key-A139612REF21-21"
},
{
"DOI": "10.3390/antiox10020272",
"doi-asserted-by": "publisher",
"key": "key-A139612REF22-22"
},
{
"author": "Mohanty RR",
"issue": "6",
"journal-title": "Europ Rev Med Pharmacol Sci.",
"key": "key-A139612REF23-23",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.2217/fmb-2020-0074",
"doi-asserted-by": "publisher",
"key": "key-A139612REF24-24"
},
{
"DOI": "10.2174/1389201021999201228212043",
"doi-asserted-by": "publisher",
"key": "key-A139612REF25-25"
},
{
"DOI": "10.1080/23744235.2021.1945675",
"doi-asserted-by": "publisher",
"key": "key-A139612REF26-26"
},
{
"DOI": "10.1186/s13063-021-05242-4",
"doi-asserted-by": "publisher",
"key": "key-A139612REF27-27"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"doi-asserted-by": "publisher",
"key": "key-A139612REF28-28"
},
{
"DOI": "10.1177/2054358118801017",
"doi-asserted-by": "publisher",
"key": "key-A139612REF29-29"
},
{
"DOI": "10.1097/01.asn.0000106780.14856.55",
"doi-asserted-by": "publisher",
"key": "key-A139612REF30-30"
},
{
"DOI": "10.1002/jmv.28393",
"doi-asserted-by": "publisher",
"key": "key-A139612REF31-31"
},
{
"author": "Bhattacharya R",
"first-page": "1",
"issue": "10",
"journal-title": "Int J Res Med Sci.",
"key": "key-A139612REF32-32",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1007/s43440-021-00296-2",
"doi-asserted-by": "publisher",
"key": "key-A139612REF33-33"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://brieflands.com/articles/jcp-139612"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of N‑Acetylcysteine in Children with Moderate COVID-19: A Placebo-Controlled Randomized Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.5812/crossmark_update_policy",
"volume": "15"
}
