Real-world efficacy of oral azvudine in hospitalized patients with COVID-19: A multicenter retrospective cohort study
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2025.102987, ChiCTR2000029853, Oct 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
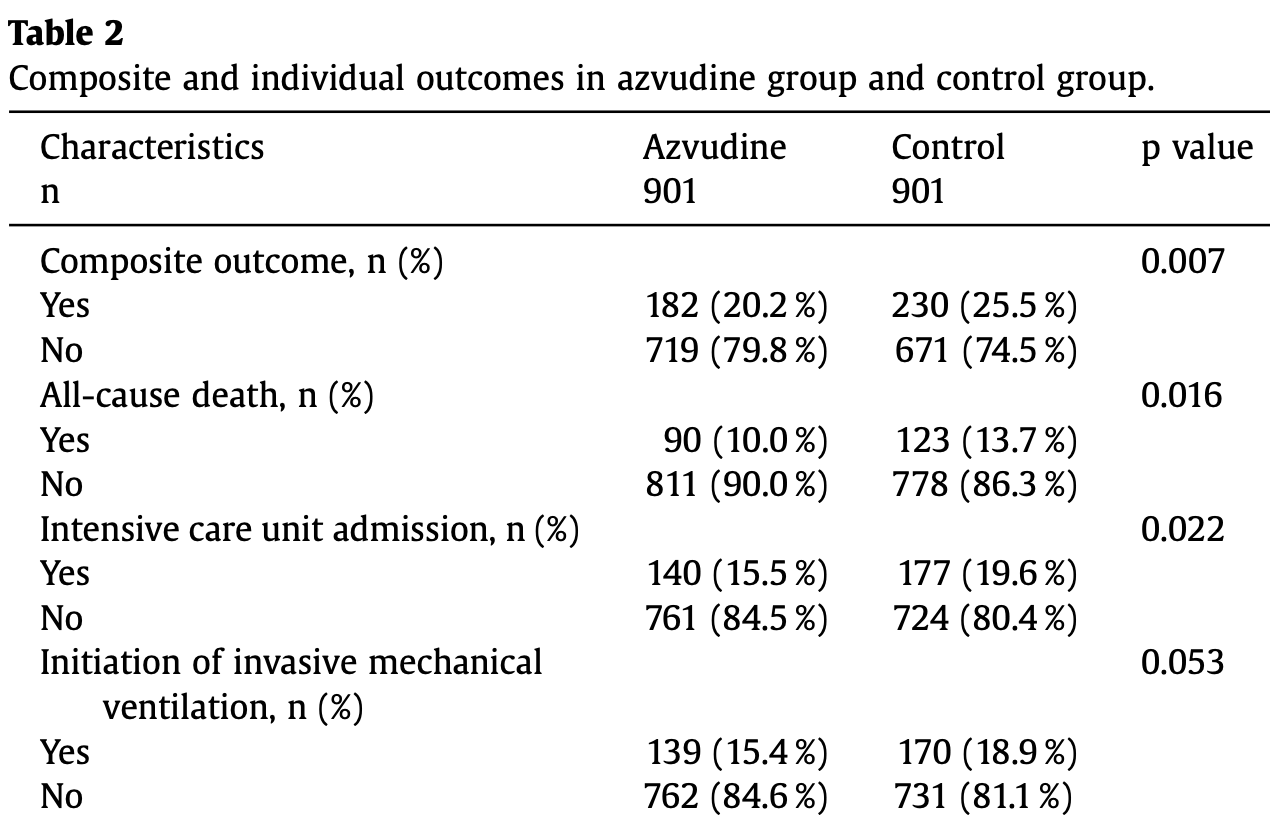
Retrospective 7,216 hospitalized COVID-19 patients in China showing reduced mortality and composite outcomes with azvudine treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 26.8% lower, RR 0.73, p = 0.02, treatment 90 of 901 (10.0%), control 123 of 901 (13.7%), NNT 27, propensity score matching.
|
|
risk of mechanical ventilation, 18.2% lower, RR 0.82, p = 0.06, treatment 139 of 901 (15.4%), control 170 of 901 (18.9%), NNT 29, propensity score matching.
|
|
risk of ICU admission, 20.9% lower, RR 0.79, p = 0.03, treatment 140 of 901 (15.5%), control 177 of 901 (19.6%), NNT 24, propensity score matching.
|
|
death/mechanical ventilation/ICU, 20.1% lower, RR 0.80, p = 0.01, treatment 182 of 901 (20.2%), control 230 of 910 (25.3%), NNT 20, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Han et al., 8 Oct 2025, retrospective, China, peer-reviewed, 7 authors, trial ChiCTR2000029853.
Contact: fghahmu@126.com, zengdxsz@126.com, wangran@ahmu.edu.cn.
Real-world efficacy of oral azvudine in hospitalized patients with COVID-19: A multicenter retrospective cohort study
Journal of Infection and Public Health, doi:10.1016/j.jiph.2025.102987
Background: Azvudine has become a widely used treatment for COVID-19 in China. Our study aimed to assess the real-world efficacy of azvudine in hospitalized COVID-19 patients during the omicron variant surge. Methods: This multicenter retrospective cohort study was conducted at three hospitals, starting from December 2022. We developed a propensity-score matching (PSM) model to compare patients receiving azvudine with a control group. The primary outcome measured was a composite outcome, while secondary outcomes included all-cause death, intensive care unit admission, and initiation of invasive mechanical ventilation. Results: We enrolled a total of 7216 hospitalized COVID-19 patients, monitoring them for 28 days. Following PSM, we included 901 patients in both the azvudine group and the control group. The incidence of the composite outcome was 20.2 % in the azvudine group and 25.5 % in the control group (p = 0.007). The allcause mortality rate was 10.0 % in the azvudine group and 13.7 % in the control group (p = 0.016). The intensive care unit admission was 15.5 % in the azvudine group and 19.6 % in the control group (p = 0.022). Conclusion: During the omicron epidemic in China, oral administration of azvudine was associated with a reduced risk of the composite outcome and all-cause mortality in COVID-19 patients.
Abbreviations severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); coronavirus disease 2019 (COVID-19); arterial partial pressure of oxygen (PaO 2 ); inspired oxygen concentration (FiO 2 ); intensive care unit (ICU); body mass index (BMI); interleukin 6 (IL-6); propensity score matching (PSM); standardized mean difference (SMD); hazard ratios (HR); confidence intervals (CI); Kaplan-Meier (KM); P-glycoprotein(P-gp).
Declaration The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Authors' contributions Rui Han was involved in the conception and design. Rui Han and Youhong Guan analyzed and interpreted the results. Pulin Li and Min Tang were responsible for drafting the paper. Daxiong Zeng critically revised its intellectual content. Guanghe Fei and Ran Wang approved the final version to be published; and that all authors agree to be accountable for all aspects of the work. All authors have approved the final article.
Ethics approval and consent to participate This study obtained approval from the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ-2023-07-34), the Ethics Committee of Hefei Second People's Hospital..
References
Alexaki, Henneicke, The role of glucocorticoids in the management of COVID-19, Horm Metab Res
Banerjee, Preissner, Preissner, Using real-world evidence data and digital monitoring to analyze the hepatotoxic profiles of biologics across more than two million patients, Sci Rep
Bland, Altman, Survival probabilities (the Kaplan-Meier method), Bmj
Bojkova, Widera, Ciesek, Wass, Michaelis et al., Reduced interferon antagonism but similar drug sensitivity in omicron variant compared to delta variant of SARS-CoV-2 isolates, Cell Res
Chang, 4'-Modified nucleosides for antiviral drug discovery: achievements and perspectives, Acc Chem Res
Chen, Guo, Deng, Wang, Gao et al., All-cause mortality in moderate and severe COVID-19 patients with myocardial injury receiving versus not receiving azvudine: a propensity score-matched analysis, Cardiol
Chen, Liu, Guo, Emerging coronaviruses: genome structure, replication, and pathogenesis, J Med Virol
Chen, Tian, Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis, Heliyon
Dal-Ré, Becker, Bottieau, Holm, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis
De Souza, Cabral, Da Silva, Arruda, Cabral et al., Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients, Front Med
Deng, Li, Sun, Zhou, Xiao, Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol
Dian, Meng, Sun, Deng, Zeng, Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities, J Infect
Faizan, Han, Lee, Policy-Driven digital health interventions for health promotion and disease prevention: a systematic review of clinical and environmental outcomes, Healthcare
Fan, Li, Zhang, Wan, Zhang et al., SARS-CoV-2 omicron variant: recent progress and future perspectives, Signal Transduct Target Ther
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Gupta, Wang, Hayek, Chan, Mathews et al., Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19, JAMA Intern Med
Haas, Angulo, Mclaughlin, Anis, Singer et al., Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data, Lancet (Lond Engl)
Han, Guan, Li, None, Journal of Infection and Public Health
Han, Guan, Li, None, Journal of Infection and Public Health
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat Rev Microbiol
Kale, Shelke, Dagar, Anders, Gaikwad, How to use COVID-19 antiviral drugs in patients with chronic kidney disease, Front Pharm
Lei, Chen, Wu, Duan, Men, Small molecules in the treatment of COVID-19, Signal Transduct Target Ther
Li, Hu, Song, High-dose but not Low-dose corticosteroids potentially delay viral shedding of patients with COVID-19, Clin Infect Dis
Li, Liu, Shen, Deng, Evaluate clinical effectiveness of azvudine with data rather than speculation, J Med Virol
Li, Wang, Lavrijsen, Lamers, De Vries et al., SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination, Cell Res
Liu, Liu, Zhang, Peng, Huang et al., Intestinal absorption mechanisms of 2′-deoxy-2′-β-fluoro-4′-azidocytidine, a cytidine analog for AIDS treatment, and its interaction with P-glycoprotein, multidrug resistance-associated protein 2 and breast cancer resistance protein, Eur J Pharm Sci
Liu, Wang, Peng, Liu, Ma et al., Effects of the antiretroviral drug 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) on P-gp, MRP2 and BCRP expressions and functions, Pharmazie
Oh, Jung, Sujata, Kim, Yon et al., Spin in randomized controlled trials of pharmacology in COVID-19: a systematic review, Acc Res
Organization, COVID-19 Therapeutic Trial Synopsis
Pan, Wang, Feng, Xu, Li et al., Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis, Lancet
Pinzón, Ortiz, Holguín, Betancur, Arango et al., Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia, PLoS One
Ren, Luo, Yu, Song, Liang et al., A randomized, Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci (Weinh)
Shang, Fu, Geng, Zhang, Zhang et al., Azvudine therapy of common COVID-19 in hemodialysis patients, J Med Virol
Sun, Dian, Shen, Zeng, Chen, Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine
Sun, Peng, Yu, Zhang, Liang et al., Mechanistic insight into antiretroviral potency of 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a Long-Lasting effect on HIV-1 prevention, J Med Chem
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant, N Engl J Med
Thompson, Stenehjem, Grannis, Ball, Naleway et al., Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings, N Engl J Med
Tsang, So, Cowling, Leung, Ip, Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study, Lancet Infect Dis
Wang, Yang, Song, Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase, Front Immunol
Yang, Wang, Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China, Eur J Med Chem
Yang, Wang, Jiang, Zhang, Zhang et al., Oral azvudine for mild-tomoderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study, J Med Virol
Yu, Chang, The first Chinese oral anti-COVID-19 drug azvudine launched, Innov (Camb)
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymushoming anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1016/j.jiph.2025.102987",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2025.102987",
"alternative-id": [
"S1876034125003363"
],
"article-number": "102987",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world efficacy of oral azvudine in hospitalized patients with COVID-19: A multicenter retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2025.102987"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Han",
"given": "Rui",
"sequence": "first"
},
{
"affiliation": [],
"family": "Guan",
"given": "Youhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Pulin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fei",
"given": "Guanghe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeng",
"given": "Daxiong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ran",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.jp",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
9
]
],
"date-time": "2025-10-09T07:53:41Z",
"timestamp": 1759996421000
},
"deposited": {
"date-parts": [
[
2025,
10,
12
]
],
"date-time": "2025-10-12T17:57:42Z",
"timestamp": 1760291862000
},
"indexed": {
"date-parts": [
[
2025,
10,
12
]
],
"date-time": "2025-10-12T18:10:39Z",
"timestamp": 1760292639781,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2025,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
1
]
],
"date-time": "2025-12-01T00:00:00Z",
"timestamp": 1764547200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
1
]
],
"date-time": "2025-12-01T00:00:00Z",
"timestamp": 1764547200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
6
]
],
"date-time": "2025-10-06T00:00:00Z",
"timestamp": 1759708800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034125003363?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034125003363?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102987",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
12
]
]
},
"published-print": {
"date-parts": [
[
2025,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1002/jmv.25681",
"article-title": "Emerging coronaviruses: genome structure, replication, and pathogenesis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "418",
"issue": "4",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2025.102987_bib1",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2025.102987_bib2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41422-022-00618-w",
"article-title": "SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "322",
"issue": "3",
"journal-title": "Cell Res",
"key": "10.1016/j.jiph.2025.102987_bib3",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00997-x",
"article-title": "SARS-CoV-2 omicron variant: recent progress and future perspectives",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "141",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jiph.2025.102987_bib4",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "141",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.jiph.2025.102987_bib5",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41422-022-00619-9",
"article-title": "Reduced interferon antagonism but similar drug sensitivity in omicron variant compared to delta variant of SARS-CoV-2 isolates",
"author": "Bojkova",
"doi-asserted-by": "crossref",
"first-page": "319",
"issue": "3",
"journal-title": "Cell Res",
"key": "10.1016/j.jiph.2025.102987_bib6",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2025.102987_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"article-title": "Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China",
"author": "Yang",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Med Chem",
"key": "10.1016/j.jiph.2025.102987_bib8",
"volume": "257",
"year": "2023"
},
{
"article-title": "The first Chinese oral anti-COVID-19 drug azvudine launched",
"author": "Yu",
"issue": "6",
"journal-title": "Innov (Camb)",
"key": "10.1016/j.jiph.2025.102987_bib9",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.3390/healthcare13182319",
"article-title": "Policy-Driven digital health interventions for health promotion and disease prevention: a systematic review of clinical and environmental outcomes",
"author": "Faizan",
"doi-asserted-by": "crossref",
"first-page": "2319",
"issue": "18",
"journal-title": "Healthcare",
"key": "10.1016/j.jiph.2025.102987_bib10",
"volume": "13",
"year": "2025"
},
{
"article-title": "A randomized, Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "10.1016/j.jiph.2025.102987_bib11",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1002/jmv.28947",
"article-title": "Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study",
"author": "Yang",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2025.102987_bib12",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"article-title": "Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study",
"author": "Sun",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jiph.2025.102987_bib13",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(23)00129-0",
"article-title": "Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "664",
"issue": "10377",
"journal-title": "Lancet",
"key": "10.1016/j.jiph.2025.102987_bib14",
"volume": "401",
"year": "2023"
},
{
"key": "10.1016/j.jiph.2025.102987_bib15",
"unstructured": "General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 in China (trial version 10). 2023 [cited 2023]Available from: 〈https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm〉."
},
{
"DOI": "10.1001/jamainternmed.2020.6252",
"article-title": "Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.jiph.2025.102987_bib16",
"volume": "181",
"year": "2021"
},
{
"key": "10.1016/j.jiph.2025.102987_bib17",
"unstructured": "Organization WH. COVID-19 Therapeutic Trial Synopsis. 2020 2020.2.18 [cited 2022 2022.02.18]Available from: 〈https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis〉."
},
{
"key": "10.1016/j.jiph.2025.102987_bib18",
"unstructured": "Propensity score matching in SPSS. 2012 [cited 2012]. Available from: 〈https://arxiv.org/abs/1201.6385〉."
},
{
"DOI": "10.1136/bmj.317.7172.1572",
"article-title": "Survival probabilities (the Kaplan-Meier method)",
"author": "Bland",
"doi-asserted-by": "crossref",
"first-page": "1572",
"issue": "7172",
"journal-title": "Bmj",
"key": "10.1016/j.jiph.2025.102987_bib19",
"volume": "317",
"year": "1998"
},
{
"DOI": "10.1002/jmv.28756",
"article-title": "Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Deng",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2025.102987_bib20",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.0c00940",
"article-title": "Mechanistic insight into antiretroviral potency of 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a Long-Lasting effect on HIV-1 prevention",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "8554",
"issue": "15",
"journal-title": "J Med Chem",
"key": "10.1016/j.jiph.2025.102987_bib21",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jiph.2025.102987_bib22",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1021/acs.accounts.1c00697",
"article-title": "4'-Modified nucleosides for antiviral drug discovery: achievements and perspectives",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "565",
"issue": "4",
"journal-title": "Acc Chem Res",
"key": "10.1016/j.jiph.2025.102987_bib23",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"article-title": "Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities",
"author": "Dian",
"doi-asserted-by": "crossref",
"first-page": "e24",
"issue": "2",
"journal-title": "J Infect",
"key": "10.1016/j.jiph.2025.102987_bib24",
"volume": "87",
"year": "2023"
},
{
"DOI": "10.1055/a-1300-2550",
"article-title": "The role of glucocorticoids in the management of COVID-19",
"author": "Alexaki",
"doi-asserted-by": "crossref",
"first-page": "9",
"issue": "1",
"journal-title": "Horm Metab Res",
"key": "10.1016/j.jiph.2025.102987_bib25",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0252057",
"article-title": "Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia",
"author": "Pinzón",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "PLoS One",
"key": "10.1016/j.jiph.2025.102987_bib26",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa829",
"article-title": "High-dose but not Low-dose corticosteroids potentially delay viral shedding of patients with COVID-19",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1297",
"issue": "7",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2025.102987_bib27",
"volume": "72",
"year": "2021"
},
{
"article-title": "Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients",
"author": "de Souza",
"journal-title": "Front Med (Lausanne)",
"key": "10.1016/j.jiph.2025.102987_bib28",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2023.e20153",
"article-title": "Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis",
"author": "Chen",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Heliyon",
"key": "10.1016/j.jiph.2025.102987_bib29",
"volume": "9",
"year": "2023"
},
{
"article-title": "Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase",
"author": "Wang",
"journal-title": "Front Immunol",
"key": "10.1016/j.jiph.2025.102987_bib30",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-01249-8",
"article-title": "Small molecules in the treatment of COVID-19",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "387",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jiph.2025.102987_bib31",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00119-0",
"article-title": "Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach",
"author": "Dal-Ré",
"doi-asserted-by": "crossref",
"first-page": "e231",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2025.102987_bib32",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41598-023-37979-0",
"article-title": "Using real-world evidence data and digital monitoring to analyze the hepatotoxic profiles of biologics across more than two million patients",
"author": "Banerjee",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2025.102987_bib33",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28926",
"article-title": "Evaluate clinical effectiveness of azvudine with data rather than speculation",
"author": "Li",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2025.102987_bib34",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"article-title": "How to use COVID-19 antiviral drugs in patients with chronic kidney disease",
"author": "Kale",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharm",
"key": "10.1016/j.jiph.2025.102987_bib35",
"volume": "14",
"year": "2023"
},
{
"article-title": "All-cause mortality in moderate and severe COVID-19 patients with myocardial injury receiving versus not receiving azvudine: a propensity score-matched analysis",
"author": "Chen",
"first-page": "103",
"issue": "2",
"journal-title": "Cardiol",
"key": "10.1016/j.jiph.2025.102987_bib36",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29007",
"article-title": "Azvudine therapy of common COVID-19 in hemodialysis patients",
"author": "Shang",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2025.102987_bib37",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2110362",
"article-title": "Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings",
"author": "Thompson",
"doi-asserted-by": "crossref",
"first-page": "1355",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2025.102987_bib38",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00947-8",
"article-title": "Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data",
"author": "Haas",
"doi-asserted-by": "crossref",
"first-page": "1819",
"issue": "10287",
"journal-title": "Lancet (Lond Engl)",
"key": "10.1016/j.jiph.2025.102987_bib39",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00732-0",
"article-title": "Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study",
"author": "Tsang",
"doi-asserted-by": "crossref",
"first-page": "421",
"issue": "4",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2025.102987_bib40",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.ejps.2017.05.009",
"article-title": "Intestinal absorption mechanisms of 2′-deoxy-2′-β-fluoro-4′-azidocytidine, a cytidine analog for AIDS treatment, and its interaction with P-glycoprotein, multidrug resistance-associated protein 2 and breast cancer resistance protein",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Eur J Pharm Sci",
"key": "10.1016/j.jiph.2025.102987_bib41",
"volume": "105",
"year": "2017"
},
{
"article-title": "Effects of the antiretroviral drug 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) on P-gp, MRP2 and BCRP expressions and functions",
"author": "Liu",
"first-page": "503",
"issue": "9",
"journal-title": "Pharmazie",
"key": "10.1016/j.jiph.2025.102987_bib42",
"volume": "73",
"year": "2018"
},
{
"DOI": "10.1080/08989621.2023.2269083",
"article-title": "Spin in randomized controlled trials of pharmacology in COVID-19: a systematic review",
"author": "Oh",
"doi-asserted-by": "crossref",
"first-page": "214",
"issue": "3",
"journal-title": "Acc Res",
"key": "10.1016/j.jiph.2025.102987_bib43",
"volume": "32",
"year": "2025"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034125003363"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-world efficacy of oral azvudine in hospitalized patients with COVID-19: A multicenter retrospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "18"
}
