Association of Azvudine with severe outcomes among hospitalized patients with COVID-19 during an omicron-dominance period in Wuhan, China: a single-center, retrospective, matched cohort study
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-11625-8, ChiCTR2300072963, Oct 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
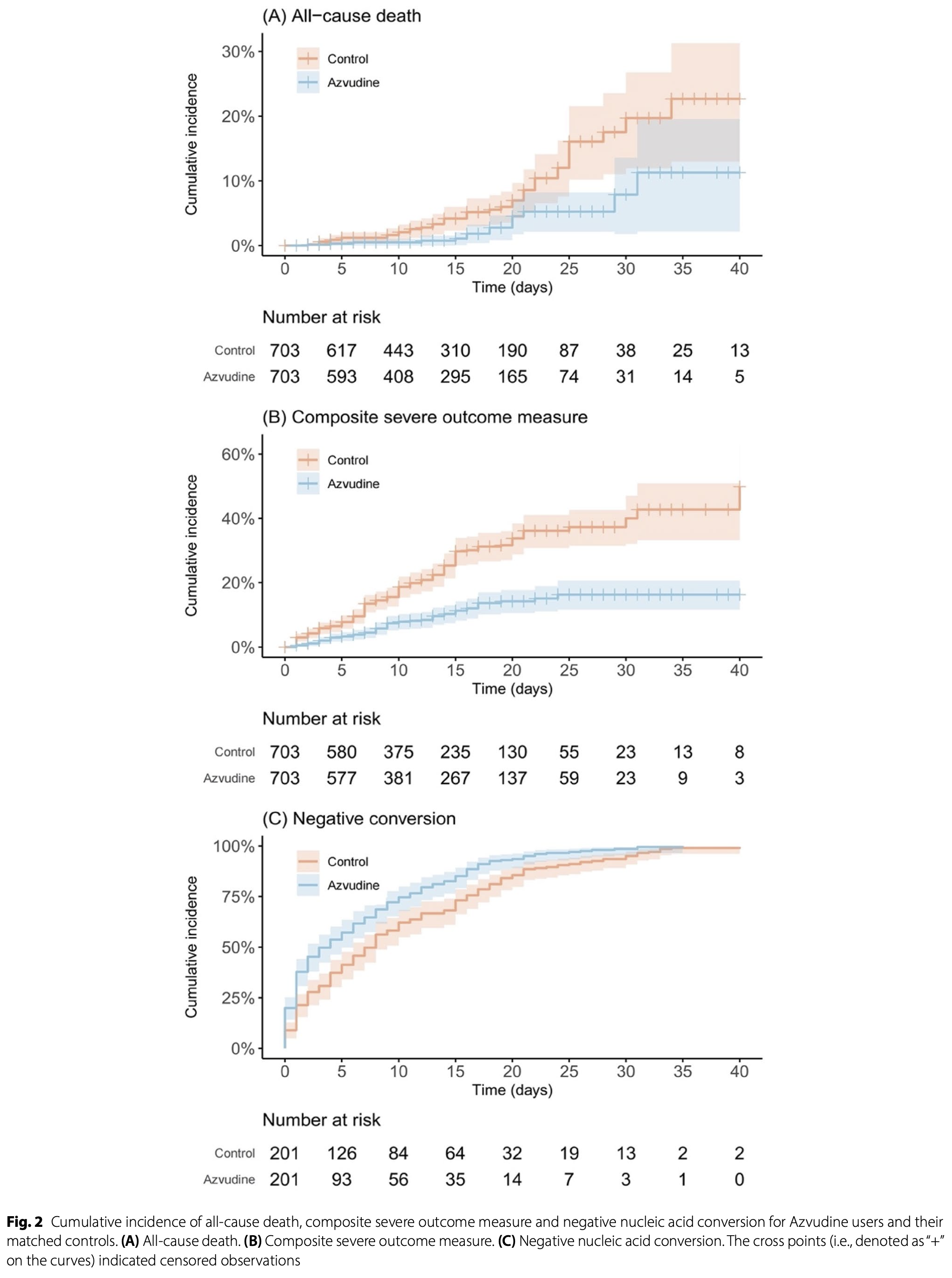
Retrospective 1,406 hospitalized COVID-19 patients (703 matched pairs) in China showing significantly lower mortality and mechanical ventilation/ICU admission, and faster viral clearance with azvudine.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 56.0% lower, HR 0.44, p = 0.005, treatment 703, control 703, propensity score matching.
|
|
ICU/mechanical ventilation, 62.0% lower, HR 0.38, p < 0.001, treatment 703, control 703, propensity score matching.
|
|
risk of no viral clearance, 34.2% lower, HR 0.66, p < 0.001, treatment 703, control 703, inverted to make HR<1 favor treatment, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Guo et al., 14 Oct 2025, retrospective, China, peer-reviewed, mean age 65.4, 12 authors, study period 1 December, 2022 - 31 May, 2023, trial ChiCTR2300072963.
Contact: sunfeng@bjmu.edu.cn, zhaoshi.cmsa@gmail.com, chenghong@znhospital.cn.
Association of Azvudine with severe outcomes among hospitalized patients with COVID-19 during an omicron-dominance period in Wuhan, China: a single-center, retrospective, matched cohort study
BMC Infectious Diseases, doi:10.1186/s12879-025-11625-8
Background Constantly emerging SARS-CoV-2 genetic variants with potent immune escape kept the COVID-19 pandemic ongoing. Apart from vaccination, effective antiviral drug is necessary to achieve clinical improvement, especially for hospitalized patients. To date, there was limited data on the clinical effectiveness of Azvudine. This study aimed to provide a comprehensive assessment of the effectiveness of Azvudine among hospitalized COVID-19 patients in a real-world healthcare setting. Method In this single-center, retrospective cohort study, hospitalized patients with laboratory-confirmed SARS-CoV-2 infection from Dec 1, 2022 to May 31, 2023 were recruited in a tertiary hospital in Wuhan, China. Azvudine recipients and controls were propensity-score matched with a ratio of 1:1, based on age, sex, baseline Charlson comorbidity index, time from symptom onset to treatment exposure, initiation of concomitant treatment at admission, and abnormality of chest computed tomography image. The primary outcomes included a composite measure of severe outcomes (ICU admission and invasive mechanical ventilation), and all-cause death, and the secondary outcome was the time to negative nucleic acid conversion. Subgroup analyses were performed according to the matching covariates. The incidence rate was computed, and the hazard ratio (HR) was estimated by using the Cox proportional hazards regression model for each outcome.
Declarations Ethics approval and consent to participate This study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (No.: 2023013 K). The need for obtaining informed consent to participate was waived by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University. This study adhered to the Declaration of Helsinki.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med
Austin, Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and monte carlo simulations, Biom J
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Cdc, COVID-19 treatments and medications
Deng, Li, Sun, Zhou, Xiao, Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol, doi:10.1002/jmv.28756
Dian, Meng, Sun, Deng, Zeng, Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities, J Infect
Dietz, Juhl, Søgaard, Reekie, Nielsen et al., Impact of age and comorbidities on SARS-CoV-2 vaccine-induced T cell immunity, Commun Med
Faraone, Qu, Evans, Zheng, Carlin et al., Neutralization escape of Omicron XBB, BR.2, and BA.2.3.20 subvariants, Cell Rep Med
Gao, Luo, Hu, Ma, Real-world effectiveness of azvudine: elixir or equivocal answer?, J Med Virol
Gao, Luo, Ren, Duan, Han et al., Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J Infect
Garibaldi, Wang, Robinson, Betz, Alexander et al., Real-world effectiveness of Remdesivir in adults hospitalized with coronavirus disease 2019 (COVID-19): A retrospective, multicenter comparative effectiveness study, Clin Infect Dis
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Link-Gelles, Ciesla, Roper, Scobie, Ali et al., Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5-and XBB/ XBB.1.5-related sublineages among immunocompetent adults -Increasing community access to testing program, united states, december 2022-january 2023, MMWR Morb Mortal Wkly Rep
Moss, The T cell immune response against SARS-CoV-2, Nat Immunol
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-Risk patients, Clin Infect Dis
Pung, Kong, Cui, Chae, Chen et al., Severity of SARS-CoV-2 Omicron XBB subvariants in Singapore, Lancet Reg Health West Pac
Ren, Luo, Yu, Song, Liang et al., A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci
Renoux, Azoulay, Suissa, Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies, Am J Epidemiol
Shen, Xiao, Sun, Li, Wu, Real-world effectiveness of azvudine in hospitalized patients with COVID-19: a retrospective cohort study, BioRxiv, doi:10.1101/2023.01.23.23284899
Sun, Dian, Shen, Zeng, Chen, Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine
Therapies, COVID-19 Treatment Guidelines
Wang, Guo, Zeng, Sun, Lu et al., Transmission characteristics and inactivated vaccine effectiveness against transmission of SARS-CoV-2 Omicron BA.5 variants in Urumqi, China, JAMA Netw Open
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong kong's Omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus Ritonavir against mortality, hospitalisation, and in-hospital outcomes among communitydwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong kong: an observational study, Lancet
Wu, Li, Shi, Chen, Jiang et al., Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19), J Intern Med
Xu, Yang, Zheng, Zhang, Cao et al., The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses, J Virol, doi:10.1128/JVI.00204-20
Yang, Wang, Jiang, Zhang, Zhang et al., Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study, J Med Virol
Ye, China's rolling COVID waves could hit every six months -infecting millions, Nature
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Ther
Yu, Chang, The first Chinese oral anti-COVID-19 drug azvudine launched, Innov (Camb)
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymushoming anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1186/s12879-025-11625-8",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-025-11625-8",
"alternative-id": [
"11625"
],
"article-number": "1325",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "10 April 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "3 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 October 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (No.: 2023013 K). The need for obtaining informed consent to participate was waived by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University. This study adhered to the Declaration of Helsinki."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Guo",
"given": "Zihao",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Wenjing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Kailu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Yun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gao",
"given": "Suyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xinping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Yanji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhai",
"given": "Ziyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Boqiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Shi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Hong",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T15:51:34Z",
"timestamp": 1760457094000
},
"deposited": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T15:51:35Z",
"timestamp": 1760457095000
},
"indexed": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T16:12:26Z",
"timestamp": 1760458346947,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
10,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T00:00:00Z",
"timestamp": 1760400000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T00:00:00Z",
"timestamp": 1760400000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-11625-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-025-11625-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-11625-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
10,
14
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
14
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "11625_CR1",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–26.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "11625_CR2",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "11625_CR3",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "11625_CR4",
"unstructured": "Butler CC, Hobbs FDR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–93.",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciab1035",
"author": "BT Garibaldi",
"doi-asserted-by": "publisher",
"first-page": "e516",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "11625_CR5",
"unstructured": "Garibaldi BT, Wang K, Robinson ML, Betz J, Caleb Alexander G, Andersen KM, et al. Real-world effectiveness of Remdesivir in adults hospitalized with coronavirus disease 2019 (COVID-19): A retrospective, multicenter comparative effectiveness study. Clin Infect Dis. 2022;75(1):e516–24.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "11625_CR6",
"unstructured": "Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022;387(9):790–8.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "11625_CR7",
"unstructured": "Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-Risk patients. Clin Infect Dis. 2023;76(3):e342–9.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "11625_CR8",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus Ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong kong: an observational study. Lancet. 2022;400(10359):1213–22.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1681",
"issue": "12",
"journal-title": "Lancet Infect Dis",
"key": "11625_CR9",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong kong’s Omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–93.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"author": "B Yu",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "11625_CR10",
"unstructured": "Yu B, Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Target Ther. 2020;5(1):236.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00204-20",
"author": "N Xu",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "11625_CR11",
"unstructured": "Xu N, Yang J, Zheng B, Zhang Y, Cao Y, Huan C, et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J Virol. 2020. https://doi.org/10.1128/JVI.00204-20.",
"year": "2020"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Z Ren",
"doi-asserted-by": "publisher",
"issue": "19",
"journal-title": "Adv Sci",
"key": "11625_CR12",
"unstructured": "Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7(19):e2001435.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "J-L Zhang",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "11625_CR13",
"unstructured": "Zhang J-L, Li Y-H, Wang L-L, Liu H-Q, Lu S-Y, Liu Y, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1101/2023.01.23.23284899",
"doi-asserted-by": "publisher",
"key": "11625_CR14",
"unstructured": "Shen M, Xiao C, Sun Y, Li D, Wu P, Jin L et al. Real-world effectiveness of azvudine in hospitalized patients with COVID-19: a retrospective cohort study. BioRxiv. 2023.Available from: https://doi.org/10.1101/2023.01.23.23284899."
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Y Sun",
"doi-asserted-by": "publisher",
"first-page": "101981",
"issue": "101981",
"journal-title": "EClinicalMedicine",
"key": "11625_CR15",
"unstructured": "Sun Y, Jin L, Dian Y, Shen M, Zeng F, Chen X, et al. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 2023;59(101981):101981.",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28947",
"author": "H Yang",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "J Med Virol",
"key": "11625_CR16",
"unstructured": "Yang H, Wang Z, Jiang C, Zhang Y, Zhang Y, Xu M, et al. Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study. J Med Virol. 2023;95(7):e28947.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28940",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "J Med Virol",
"key": "11625_CR17",
"unstructured": "Gao Y, Luo Z, Hu Z, Ma Y. Real-world effectiveness of azvudine: elixir or equivocal answer? J Med Virol. 2023;95(7):e28940.",
"volume": "95",
"year": "2023"
},
{
"key": "11625_CR18",
"unstructured": "CDC. COVID-19 treatments and medications. Centers for Disease Control and Prevention. 2023 [cited 2023 Aug 7]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html"
},
{
"DOI": "10.1038/d41586-023-01872-7",
"author": "Y Ye",
"doi-asserted-by": "publisher",
"first-page": "442",
"issue": "7965",
"journal-title": "Nature",
"key": "11625_CR19",
"unstructured": "Ye Y. China’s rolling COVID waves could hit every six months - infecting millions. Nature. 2023;618(7965):442–3.",
"volume": "618",
"year": "2023"
},
{
"key": "11625_CR20",
"unstructured": "Transcript of the press conference of the Joint Prevention and Control Mechanism of the State Council on December 7. 2022. Gov.cn. [cited 2023 Aug 7]. Available from: http://www.nhc.gov.cn/xcs/s3574/202212/0001cc3efcce46e8aad5c4f40c66da55.shtml"
},
{
"key": "11625_CR21",
"unstructured": "Notice on Printing and Distributing the Diagnosis and Treatment Plan for Novel Coronavirus Infection. (Trial Version 10). Gov.cn. [cited 2023 Aug 7]. Available from: https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm"
},
{
"DOI": "10.1093/aje/kwab028",
"author": "C Renoux",
"doi-asserted-by": "publisher",
"first-page": "1452",
"issue": "8",
"journal-title": "Am J Epidemiol",
"key": "11625_CR22",
"unstructured": "Renoux C, Azoulay L, Suissa S. Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies. Am J Epidemiol. 2021;190(8):1452–6.",
"volume": "190",
"year": "2021"
},
{
"DOI": "10.1002/bimj.200810488",
"author": "PC Austin",
"doi-asserted-by": "publisher",
"first-page": "171",
"issue": "1",
"journal-title": "Biom J",
"key": "11625_CR23",
"unstructured": "Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and monte carlo simulations. Biom J. 2009;51(1):171–84.",
"volume": "51",
"year": "2009"
},
{
"DOI": "10.1016/j.xcrm.2023.101049",
"author": "JN Faraone",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Cell Rep Med",
"key": "11625_CR24",
"unstructured": "Faraone JN, Qu P, Evans JP, Zheng Y-M, Carlin C, Anghelina M, et al. Neutralization escape of Omicron XBB, BR.2, and BA.2.3.20 subvariants. Cell Rep Med. 2023;4(5):101049.",
"volume": "4",
"year": "2023"
},
{
"author": "R Pung",
"first-page": "100849",
"issue": "100849",
"journal-title": "Lancet Reg Health West Pac",
"key": "11625_CR25",
"unstructured": "Pung R, Kong XP, Cui L, Chae S-R, Chen MI-C, Lee VJ, et al. Severity of SARS-CoV-2 Omicron XBB subvariants in Singapore. Lancet Reg Health West Pac. 2023;37(100849):100849.",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7205e1",
"author": "R Link-Gelles",
"doi-asserted-by": "publisher",
"first-page": "119",
"issue": "5",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "11625_CR26",
"unstructured": "Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults - Increasing community access to testing program, united states, december 2022-january 2023. MMWR Morb Mortal Wkly Rep. 2023;72(5):119–24.",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2023.5755",
"author": "K Wang",
"doi-asserted-by": "publisher",
"first-page": "e235755",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "11625_CR27",
"unstructured": "Wang K, Guo Z, Zeng T, Sun S, Lu Y, Wang J, et al. Transmission characteristics and inactivated vaccine effectiveness against transmission of SARS-CoV-2 Omicron BA.5 variants in Urumqi, China. JAMA Netw Open. 2023;6(3):e235755.",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1111/joim.13063",
"author": "J Wu",
"doi-asserted-by": "publisher",
"first-page": "128",
"issue": "1",
"journal-title": "J Intern Med",
"key": "11625_CR28",
"unstructured": "Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020;288(1):128–38.",
"volume": "288",
"year": "2020"
},
{
"key": "11625_CR29",
"unstructured": "Therapies. COVID-19 Treatment Guidelines. [cited 2023 Aug 8]. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/"
},
{
"DOI": "10.1002/jmv.28756",
"author": "G Deng",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "11625_CR30",
"unstructured": "Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, et al. Real-world effectiveness of azvudine versus nirmatrelvir–ritonavir in hospitalized patients with COVID‐19: a retrospective cohort study. J Med Virol. 2023. https://doi.org/10.1002/jmv.28756.",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"author": "Y Dian",
"doi-asserted-by": "publisher",
"first-page": "e24",
"issue": "2",
"journal-title": "J Infect",
"key": "11625_CR31",
"unstructured": "Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Infect. 2023;87(2):e24-7.",
"volume": "87",
"year": "2023"
},
{
"author": "B Yu",
"issue": "6",
"journal-title": "Innov (Camb)",
"key": "11625_CR32",
"unstructured": "Yu B, Chang J. The first Chinese oral anti-COVID-19 drug azvudine launched. Innov (Camb). 2022;3(6):100321.",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"first-page": "e158",
"issue": "6",
"journal-title": "J Infect",
"key": "11625_CR33",
"unstructured": "Gao Y, Luo Z, Ren S, Duan Z, Han Y, Liu H, et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 2023;86(6):e158–60.",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1038/s43856-023-00277-x",
"author": "LL Dietz",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Commun Med",
"key": "11625_CR34",
"unstructured": "Dietz LL, Juhl AK, Søgaard OS, Reekie J, Nielsen H, Johansen IS, et al. Impact of age and comorbidities on SARS-CoV-2 vaccine-induced T cell immunity. Commun Med. 2023;3(1):58.",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1038/s41590-021-01122-w",
"author": "P Moss",
"doi-asserted-by": "publisher",
"first-page": "186",
"issue": "2",
"journal-title": "Nat Immunol",
"key": "11625_CR35",
"unstructured": "Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–93.",
"volume": "23",
"year": "2022"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-025-11625-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Association of Azvudine with severe outcomes among hospitalized patients with COVID-19 during an omicron-dominance period in Wuhan, China: a single-center, retrospective, matched cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}