The efficacy of Zafirlukast as a SARS-CoV-2 helicase inhibitor in adult patients with moderate COVID-19 Pneumonia (pilot randomized clinical trial)
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.11.016, Dec 2022
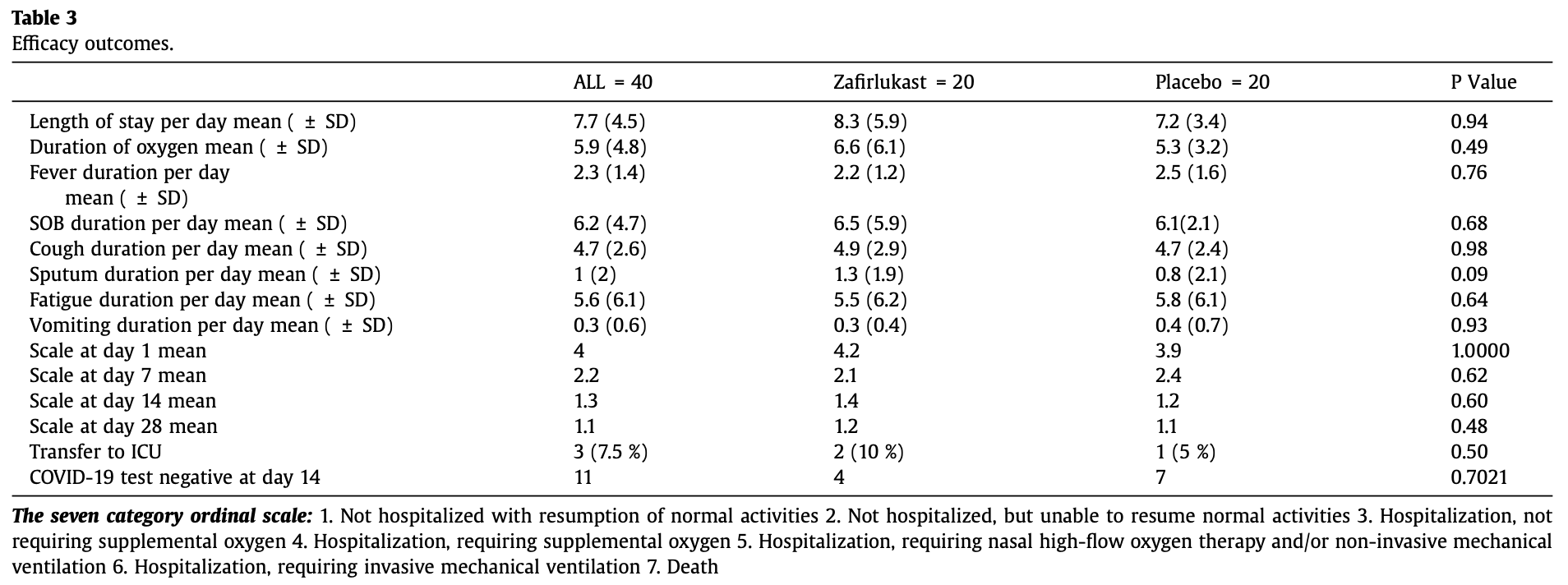
RCT 40 hospitalized patients with moderate COVID-19 pneumonia showing no significant difference with zafirlukast treatment compared to placebo. Authors suggest that the lack of efficacy might be related to timing of drug administration relative to symptom onset or insufficient dosing.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
|
risk of ICU admission, 100% higher, RR 2.00, p = 1.00, treatment 2 of 20 (10.0%), control 1 of 20 (5.0%).
|
|
risk of 7-point scale, 9.1% higher, RR 1.09, p = 0.48, treatment 20, control 20, day 28.
|
|
risk of 7-point scale, 16.7% higher, RR 1.17, p = 0.60, treatment 20, control 20, day 14.
|
|
risk of 7-point scale, 12.5% lower, RR 0.88, p = 0.62, treatment 20, control 20, day 7.
|
|
hospitalization time, 15.3% higher, relative time 1.15, p = 0.47, treatment mean 8.3 (±5.9) n=20, control mean 7.2 (±3.4) n=20.
|
|
risk of no viral clearance, 23.1% higher, RR 1.23, p = 0.48, treatment 16 of 20 (80.0%), control 13 of 20 (65.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ghobain et al., 31 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Saudi Arabia, peer-reviewed, 11 authors, study period April 2021 - September 2021.
Contact: alanezi@hotmail.com.
The efficacy of Zafirlukast as a SARS-CoV-2 helicase inhibitor in adult patients with moderate COVID-19 Pneumonia (pilot randomized clinical trial)
Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.11.016
Objective: To assess the efficacy of Zafirlukast as a SARS-CoV-2 Helicase Inhibitor in adult patients with moderate COVID-19 symptoms (hospitalized patients with COVID-19 pneumonia who were not admitted to an intensive care unit). Methods: We conducted a randomized, double blind, placebo-controlled, pilot trial with adult patients with moderate COVID-19 pneumonia. The sample was randomized to Zafirlukast 10 mg BD for 10 days plus standard care vs placebo plus standard care. The primary outcome was the complete resolution of all symptoms. The secondary outcomes were the duration of oxygen therapy, and length of hospital stay (LOS). Results: In total, 40 patients were randomized (20 to Zafirlukast and 20 to the control). The time to the resolution of clinical symptoms in both groups was not significantly different. Regarding the fever, 0.3 days [95 % CI, -1.19, 0.69], p = 0.76, for shortness of breath, the difference was 0.4 days [95 % CI, -2.67, 3.46], p = 0.68, for cough the difference was 0.2 days [95 % CI, -1.45, 1.95], p = 0.98, for sputum the difference was 0.5 days [95 % CI, -0.75, 1.85], p = 0.09, for vomiting the difference was 0.1 days [95 % CI, -0.50, 0.30], p = 0.93, for fatigue the difference was 0.3 days [95 % CI, -4.32, 3.62], p = 0.64. The LOS per day for the two groups was not significantly different, 1.1 days [95 % CI,-2.03, 4.28], p = 0.94, nor was the duration of oxygen therapy per days, 1.3 days [95 % CI, 4.49], p = 0.49. Regarding the 7 category ordinary scale, there was no significant difference between the two groups at day 7 (p-value = 0.62), day 14 (p-value = 0.60) and day 28 (p-value = 0.48). Conclusion: Among adult patients hospitalized with COVID-19 pneumonia, the treatment with Zafirlukast, compared to placebo, did not significantly improve symptoms resolution.
Ethical approval The trial was approved by Institutional Review Board of King Abdullah International Medical Research Center and the Saudi Food and Drug Authority.
Competing interests None declared
References
Adedeji, Lazarus, Biochemical characterization of middle east respiratory syndrome coronavirus helicase e00235-16, mSphere
Arabi, Alothman, Balkhy, Al-Dawood, Aljohani et al., Treatment of middle east respiratory syndrome with a combination of lopinavirritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial, Trials
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2019014
Chan, Lai, Chu, Tsui, Tam et al., Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study, Hong Kong Med J
Fu, Han, Zhu, Bai, Yi et al., Risk factors for viral RNA shedding in COVID-19 patients, Eur Respir J
He, Deng, Li, Coronavirus disease 2019: what we know?, J Med Virol, doi:10.1002/jmv.25766
He, Lau, Wu, Deng, Wang et al., Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med, doi:10.1038/s41591-020-0869-5
Horby, Mafham, Linsell, Effect of hydroxychloroquine in hospitalized patients with COVID-19, N Engl J Med
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Ivanov, Thiel, Dobbe, Van Der Meer, Snijder et al., Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase, J Virol
Jang, Lee, Yeo, Jeong, Kim, Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase, Biochem Biophys Res Commun
Martinez, Espinosa, Adamek, Thomas, Chau et al., Breathing new life into West Nile virus therapeutics; discovery and study of zafirlukast as an NS2B-NS3 protease inhibitor, Eur J Med Chem, doi:10.1016/j.ejmech.2018.08.077
Mehyar, Mashhour, Islam, Alhadrami, Tolah et al., Discovery of Zafirlukast as a novel SARS-CoV-2 helicase inhibitor using in silico modelling and a FRET-based assay, SAR and QSAR in Environmental Research
Self, Semler, Leither, Casey, Angus et al., Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.22240
Shum, Tanner, Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase, Chembiochem
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transpl
Singhal, A review of coronavirus disease-2019 (COVID-19), Indian J Pedia, doi:10.1007/s12098-020-03263-6
Tanner, Watt, Chai, Lu, Peiris, The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases, J Biol Chem
Yamamoto, Yang, Yoshinaka, Amari, Nakano et al., HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus, Biochem Biophys Res Commun
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.1016/j.jiph.2022.11.016",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2022.11.016",
"alternative-id": [
"S1876034122003094"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "The efficacy of Zafirlukast as a SARS-CoV-2 helicase inhibitor in adult patients with moderate COVID-19 Pneumonia (pilot randomized clinical trial)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2022.11.016"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Ghobain",
"given": "M.Al",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rebh",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saad",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "A.H.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4977-2111",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mehyar",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mashhour",
"given": "A.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3855-5354",
"affiliation": [],
"authenticated-orcid": false,
"family": "Islam",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alobaida",
"given": "Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alaskar",
"given": "A.S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boudjelal",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeraisy",
"given": "M.Al",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T04:13:25Z",
"timestamp": 1668658405000
},
"deposited": {
"date-parts": [
[
2024,
5,
29
]
],
"date-time": "2024-05-29T22:58:28Z",
"timestamp": 1717023508000
},
"funder": [
{
"DOI": "10.13039/501100013302",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100013302",
"id-type": "DOI"
}
],
"name": "King Abdullah International Medical Research Center"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T15:49:30Z",
"timestamp": 1740152970638,
"version": "3.37.3"
},
"is-referenced-by-count": 3,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
14
]
],
"date-time": "2022-11-14T00:00:00Z",
"timestamp": 1668384000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034122003094?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034122003094?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1546-1550",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1002/jmv.25766",
"article-title": "Coronavirus disease 2019: what we know?",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "719",
"issue": "7",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2022.11.016_bib1",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1007/s12098-020-03263-6",
"article-title": "A review of coronavirus disease-2019 (COVID-19)",
"author": "Singhal",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "4",
"journal-title": "Indian J Pedia",
"key": "10.1016/j.jiph.2022.11.016_bib2",
"volume": "87",
"year": "2020"
},
{
"DOI": "10.1080/1062936X.2021.1993995",
"article-title": "Discovery of Zafirlukast as a novel SARS-CoV-2 helicase inhibitor using in silico modelling and a FRET-based assay",
"author": "Mehyar",
"doi-asserted-by": "crossref",
"first-page": "963",
"issue": "12",
"journal-title": "SAR and QSAR in Environmental Research",
"key": "10.1016/j.jiph.2022.11.016_bib3",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1128/mSphere.00235-16",
"article-title": "Biochemical characterization of middle east respiratory syndrome coronavirus helicase",
"author": "Adedeji",
"doi-asserted-by": "crossref",
"journal-title": "mSphere",
"key": "10.1016/j.jiph.2022.11.016_bib4",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1128/JVI.78.11.5619-5632.2004",
"article-title": "Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase",
"author": "Ivanov",
"doi-asserted-by": "crossref",
"first-page": "5619",
"journal-title": "J Virol",
"key": "10.1016/j.jiph.2022.11.016_bib5",
"volume": "78",
"year": "2004"
},
{
"DOI": "10.1074/jbc.C300328200",
"article-title": "The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases",
"author": "Tanner",
"doi-asserted-by": "crossref",
"first-page": "39578",
"journal-title": "J Biol Chem",
"key": "10.1016/j.jiph.2022.11.016_bib6",
"volume": "278",
"year": "2003"
},
{
"DOI": "10.1016/j.ejmech.2018.08.077",
"article-title": "Breathing new life into West Nile virus therapeutics; discovery and study of zafirlukast as an NS2B-NS3 protease inhibitor",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "1202",
"journal-title": "Eur J Med Chem",
"key": "10.1016/j.jiph.2022.11.016_bib7",
"volume": "157",
"year": "2018"
},
{
"key": "10.1016/j.jiph.2022.11.016_bib8",
"unstructured": "World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis 2020a, available at https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis."
},
{
"DOI": "10.1002/cbic.200800491",
"article-title": "Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase",
"author": "Shum",
"doi-asserted-by": "crossref",
"first-page": "3037",
"journal-title": "Chembiochem",
"key": "10.1016/j.jiph.2022.11.016_bib9",
"volume": "9",
"year": "2008"
},
{
"DOI": "10.1016/j.bbrc.2007.12.020",
"article-title": "Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "738",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.jiph.2022.11.016_bib10",
"volume": "366",
"year": "2008"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19",
"author": "Cavalcanti",
"doi-asserted-by": "crossref",
"first-page": "2041",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.11.016_bib11",
"volume": "383",
"year": "2020"
},
{
"key": "10.1016/j.jiph.2022.11.016_bib12",
"unstructured": "Horby P., Mafham M., Linsell L., et al.; RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020."
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "2165",
"issue": "21",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2022.11.016_bib13",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jiph.2022.11.016_bib14",
"unstructured": "Zou L., Ruan F., Huang M., Liang L. , Huang H. , Hong Z. , et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. DOI: 10.1056/NEJMc2001737."
},
{
"DOI": "10.1183/13993003.01190-2020",
"article-title": "Risk factors for viral RNA shedding in COVID-19 patients",
"author": "Fu",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "10.1016/j.jiph.2022.11.016_bib15",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Nat Med",
"key": "10.1016/j.jiph.2022.11.016_bib16",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal",
"author": "Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "405",
"journal-title": "J Heart Lung Transpl",
"key": "10.1016/j.jiph.2022.11.016_bib17",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2004.04.083",
"article-title": "HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.jiph.2022.11.016_bib18",
"year": "2004"
},
{
"article-title": "Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study",
"author": "Chan",
"journal-title": "Hong Kong Med J",
"key": "10.1016/j.jiph.2022.11.016_bib19",
"year": "2003"
},
{
"DOI": "10.1186/s13063-017-2427-0",
"article-title": "Treatment of middle east respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial",
"author": "Arabi",
"doi-asserted-by": "crossref",
"journal-title": "Trials",
"key": "10.1016/j.jiph.2022.11.016_bib20",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"journal-title": "Lancet",
"key": "10.1016/j.jiph.2022.11.016_bib21",
"volume": "395",
"year": "2020"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034122003094"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The efficacy of Zafirlukast as a SARS-CoV-2 helicase inhibitor in adult patients with moderate COVID-19 Pneumonia (pilot randomized clinical trial)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "15"
}