COVID-19 and Kidney Transplantation: The impact of remdesivir on renal function and outcome - a retrospective cohort study
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.03.015, Mar 2022
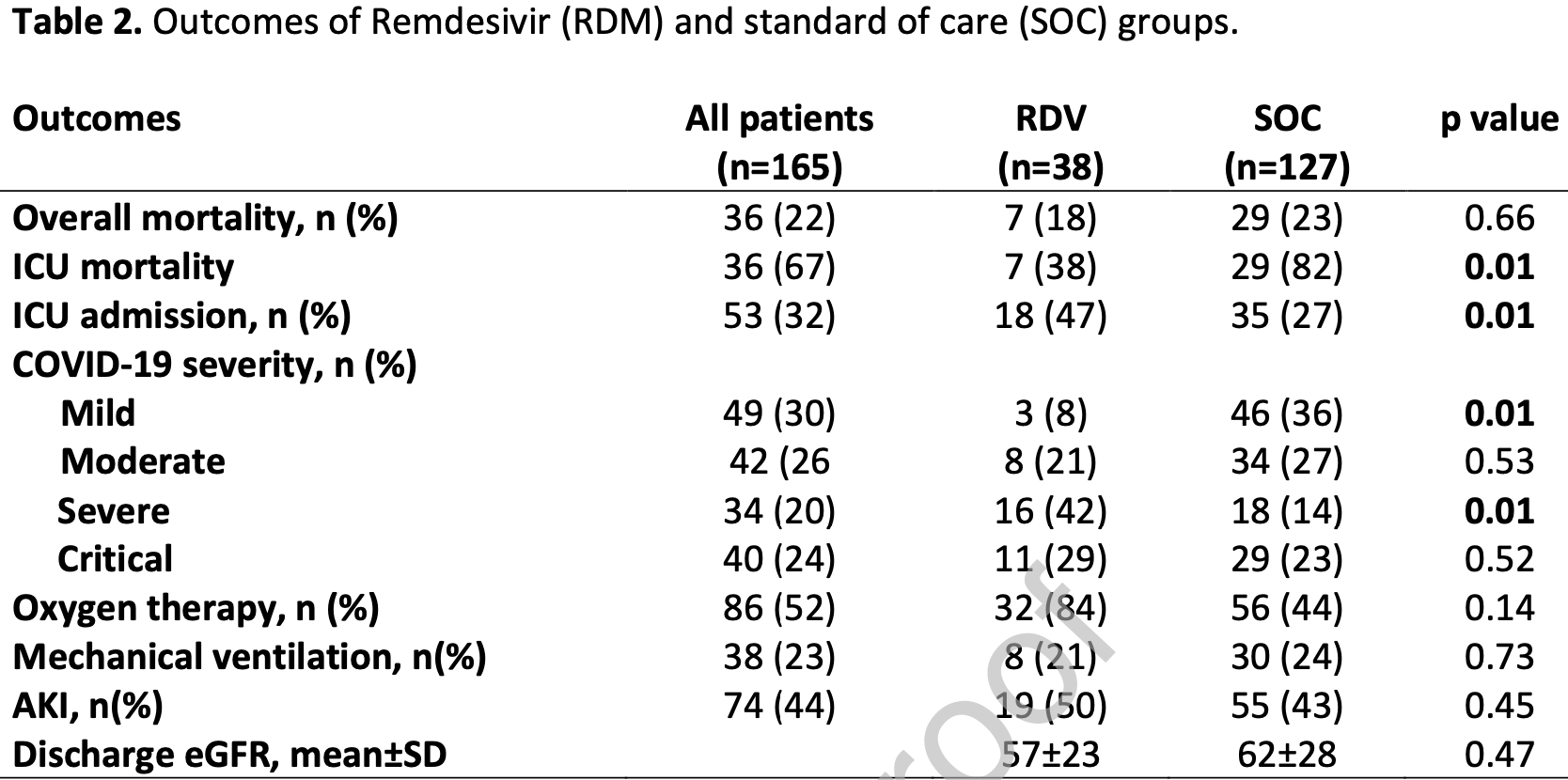
Retrospective 165 hospitalized COVID-19+ kidney transplant patients, 38 treated with remdesivir, showing no significant difference in mortality, higher ICU admission, and lower ICU mortality. Subject to confounding by time with significant changes to SOC and treatment propensity during the study period.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
This study is excluded in the after exclusion results of meta-analysis:
substantial confounding by time possible due to significant changes in SOC and treatment propensity during the study period.
|
risk of death, 19.3% lower, RR 0.81, p = 0.66, treatment 7 of 38 (18.4%), control 29 of 127 (22.8%), NNT 23.
|
|
risk of mechanical ventilation, 10.9% lower, RR 0.89, p = 0.73, treatment 8 of 38 (21.1%), control 30 of 127 (23.6%), NNT 39.
|
|
risk of ICU admission, 71.9% higher, RR 1.72, p = 0.01, treatment 18 of 38 (47.4%), control 35 of 127 (27.6%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Elec et al., 14 Mar 2022, retrospective, Romania, peer-reviewed, 9 authors, study period 1 March, 2020 - 31 May, 2021.
COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.03.015
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST There are no conflicts of interests to be reported
References
Adamsick, Gandhi, Bidell, Elshaboury, Bhattacharyya et al., Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19, J Am Soc Nephrol
Beigel, Tomashek, Dodd, Mehta, Zingman et al., ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Benfield, Bodilsen, Brieghel, Harboe, Helleberg et al., Improved survival among hospitalized patients with COVID-19 treated with remdesivir and dexamethasone. A nationwide population-based cohort study, Clin Infect Dis, doi:10.1093/cid/ciab536
Buxeda, Arias-Cabrales, Pérez-Sáez, Cacho, Pelegrin et al., Use and Safety of Remdesivir in Kidney Transplant Recipients With COVID-19, Kidney Int Rep
Chen, Kuo, Lee, Yang, Wu et al., Incidence of Mortality, Acute Kidney Injury and Graft Loss in Adult Kidney Transplant Recipients with Coronavirus Disease 2019: Systematic Review and Meta-Analysis, J Clin Med
Deb, Reeves, Hopefl, Bejusca, ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential, Pharmaceuticals
Diaz, Christensen, Pusch, Goulet, Chang et al., Remdesivir and Mortality in Patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciab698
Elec, Bolboacă, Muntean, Elec, Cismaru et al., Comparing the First and Second Wave of COVID-19 in Kidney Transplant Recipients: An East-European Perspective, Eur Surg Res, doi:10.1159/000517559
Elec, Oltean, Goldis, Cismaru, Lupse et al., COVID-19 after kidney transplantation: Early outcomes and renal function following antiviral treatment, Int J Infect Dis
Elens, Langman, Hesselink, Bergan, Moes et al., Pharmacologic Treatment of Transplant Recipients Infected With SARS-CoV-2: Considerations Regarding Therapeutic Drug Monitoring and Drug-Drug Interactions, Ther Drug Monit
Garcia, Alonso, Camon, Cardozo, Albiach et al., Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19
Goldman, Lye, Hui, Marks, Bruno et al., -540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19, N Engl J Med
Group, Elm, Altman, Egger, Pocock et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, Am J Transplant, doi:10.1016/S0140-6736(07)61602-X
Harvey, Morgan, Cancer, inflammation, and therapy: effects on cytochrome p450-mediated drug metabolism and implications for novel immunotherapeutic agents, Clin Pharmacol Ther
Heldman, Kates, Safa, Kotton, Georgia et al., Changing Trends in Mortality Among Solid Organ Transplant Recipients Hospitalized for Covid-19 During the Course of the Pandemic, Am J Transplant, doi:10.1111/ajt.16840
Jager, Kramer, Chesnaye, Couchoud, Sánchez-Álvarez et al., Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe, Kidney Int
Jayant, Reccia, Bachul, Al-Salmay, Pyda et al., The Impact of COVID-19 on Kidney Transplant Recipients in Pre-Vaccination and Delta Strain Era: A Systematic Review and Meta-Analysis, J Clin Med
Lo, Jordan, Arvey, Sudhamsu, Shrivastava-Ranjan et al., GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses, Sci Rep
Luke, Tomaszewski, Damle, Schlamm, Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD), J Pharm Sci, doi:10.1002/jps.22109
Maggiore, Abramowicz, Crespo, Mariat, Mjoen et al., How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion, Nephrol Dial Transplant
Martínez, Salas, Ballestín, Antiviral Therapeutic Approaches for SARS-CoV-2 Infection: A Systematic Review, Pharmaceuticals
Meshram, Kute, Patel, Banerjee, Navadiya et al., Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: A retrospective cohort from a developing nation, Transpl Infect Dis
Olender, Perez, Go, Balani, Eg et al., GS-US-540-5773 and GS-US-540-5807 Investigators. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care, Clin Infect Dis
Oltean, Søfteland, Bagge, Ekelund, Felldin et al., Covid-19 in kidney transplant recipients: a systematic review of the case series available three months into the pandemic, Infect Dis (Lond)
Oltean, Ţăţulescu, Bondor, Slavcovici, Cismaru et al., Charlson's weighted index of comorbidities is useful in assessing the risk of death in septic patients, J Crit Care
Qin, Moore, Anjan, Rahamimov, Sifri et al., Risk of Breakthrough SARS-CoV-2
Spinner, Gottlieb, Criner, López, Cattelan et al., Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.16349
Villanego, Mazuecos, Pérez-Flores, Moreso, Andrés et al., Spanish Society of Nephrology COVID-19
DOI record:
{
"DOI": "10.1016/j.ijid.2022.03.015",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2022.03.015",
"alternative-id": [
"S1201971222001515"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2022.03.015"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Elec",
"given": "Florin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5630-0155",
"affiliation": [],
"authenticated-orcid": false,
"family": "Magnusson",
"given": "Jesper",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elec",
"given": "Alina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muntean",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Antal",
"given": "Oana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moisoiu",
"given": "Tudor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cismaru",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lupse",
"given": "Mihaela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3783-5207",
"affiliation": [],
"authenticated-orcid": false,
"family": "Oltean",
"given": "Mihai",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
14
]
],
"date-time": "2022-03-14T21:14:27Z",
"timestamp": 1647292467000
},
"deposited": {
"date-parts": [
[
2023,
2,
17
]
],
"date-time": "2023-02-17T03:38:38Z",
"timestamp": 1676605118000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T11:52:41Z",
"timestamp": 1712577161554
},
"is-referenced-by-count": 15,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
10
]
],
"date-time": "2022-03-10T00:00:00Z",
"timestamp": 1646870400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222001515?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222001515?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "247-253",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.03.015_bib0002",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.ekir.2021.06.023",
"article-title": "Use and Safety of Remdesivir in Kidney Transplant Recipients With COVID-19",
"author": "Buxeda",
"doi-asserted-by": "crossref",
"first-page": "2305",
"issue": "9",
"journal-title": "Kidney Int Rep",
"key": "10.1016/j.ijid.2022.03.015_bib0004",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/jcm10215162",
"article-title": "Incidence of Mortality, Acute Kidney Injury and Graft Loss in Adult Kidney Transplant Recipients with Coronavirus Disease 2019: Systematic Review and Meta-Analysis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "5162",
"issue": "21",
"journal-title": "J Clin Med",
"key": "10.1016/j.ijid.2022.03.015_bib0005",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/ph14070655",
"article-title": "ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential",
"author": "Deb",
"doi-asserted-by": "crossref",
"first-page": "655",
"issue": "7",
"journal-title": "Pharmaceuticals (Basel)",
"key": "10.1016/j.ijid.2022.03.015_bib0006",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.01.023",
"article-title": "COVID-19 after kidney transplantation: Early outcomes and renal function following antiviral treatment",
"author": "Elec",
"doi-asserted-by": "crossref",
"first-page": "426",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijid.2022.03.015_bib0008",
"volume": "104",
"year": "2021"
},
{
"article-title": "Comparing the First and Second Wave of COVID-19 in Kidney Transplant Recipients: An East-European Perspective",
"author": "Elec",
"first-page": "298",
"journal-title": "Eur Surg Res",
"key": "10.1016/j.ijid.2022.03.015_bib0009",
"year": "2021"
},
{
"DOI": "10.1097/FTD.0000000000000761",
"article-title": "Pharmacologic Treatment of Transplant Recipients Infected With SARS-CoV-2: Considerations Regarding Therapeutic Drug Monitoring and Drug-Drug Interactions",
"author": "Elens",
"doi-asserted-by": "crossref",
"first-page": "360",
"issue": "3",
"journal-title": "Ther Drug Monit",
"key": "10.1016/j.ijid.2022.03.015_bib0010",
"volume": "42",
"year": "2020"
},
{
"key": "10.1016/j.ijid.2022.03.015_bib0011",
"series-title": "Summary on compassionate use",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.03.015_bib0014",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/clpt.2014.143",
"article-title": "Cancer, inflammation, and therapy: effects on cytochrome p450-mediated drug metabolism and implications for novel immunotherapeutic agents",
"author": "Harvey",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "4",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ijid.2022.03.015_bib0015",
"volume": "96",
"year": "2014"
},
{
"article-title": "Changing Trends in Mortality Among Solid Organ Transplant Recipients Hospitalized for Covid-19 During the Course of the Pandemic",
"author": "Heldman",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ijid.2022.03.015_bib0016",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2020.09.006",
"article-title": "Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe",
"author": "Jager",
"doi-asserted-by": "crossref",
"first-page": "1540",
"issue": "6",
"journal-title": "Kidney Int",
"key": "10.1016/j.ijid.2022.03.015_bib0018",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.3390/jcm10194533",
"article-title": "The Impact of COVID-19 on Kidney Transplant Recipients in Pre-Vaccination and Delta Strain Era: A Systematic Review and Meta-Analysis",
"author": "Jayant",
"doi-asserted-by": "crossref",
"first-page": "4533",
"issue": "19",
"journal-title": "J Clin Med",
"key": "10.1016/j.ijid.2022.03.015_bib0019",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/srep43395",
"article-title": "GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses",
"author": "Lo",
"doi-asserted-by": "crossref",
"first-page": "43395",
"journal-title": "Sci Rep.",
"key": "10.1016/j.ijid.2022.03.015_bib0020",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1002/jps.22109",
"article-title": "Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD)",
"author": "Luke",
"doi-asserted-by": "crossref",
"first-page": "3291",
"issue": "8",
"journal-title": "J Pharm Sci",
"key": "10.1016/j.ijid.2022.03.015_bib0021",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.1093/ndt/gfaa130",
"article-title": "How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion",
"author": "Maggiore",
"doi-asserted-by": "crossref",
"first-page": "899",
"issue": "6",
"journal-title": "Nephrol Dial Transplant",
"key": "10.1016/j.ijid.2022.03.015_bib0022",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1111/tid.13629",
"article-title": "Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: A retrospective cohort from a developing nation",
"author": "Meshram",
"doi-asserted-by": "crossref",
"first-page": "e13629",
"issue": "4",
"journal-title": "Transpl Infect Dis",
"key": "10.1016/j.ijid.2022.03.015_bib0023",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1041",
"article-title": "Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care",
"author": "Olender",
"doi-asserted-by": "crossref",
"first-page": "e4166",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2022.03.015_bib0024",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1080/23744235.2020.1792977",
"article-title": "Covid-19 in kidney transplant recipients: a systematic review of the case series available three months into the pandemic",
"author": "Oltean",
"doi-asserted-by": "crossref",
"first-page": "830",
"issue": "11",
"journal-title": "Infect Dis (Lond)",
"key": "10.1016/j.ijid.2022.03.015_bib0025",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2011.08.021",
"article-title": "Charlson's weighted index of comorbidities is useful in assessing the risk of death in septic patients",
"author": "Oltean",
"doi-asserted-by": "crossref",
"first-page": "370",
"issue": "4",
"journal-title": "J Crit Care",
"key": "10.1016/j.ijid.2022.03.015_bib0026",
"volume": "27",
"year": "2012"
},
{
"DOI": "10.1097/TP.0000000000003907",
"article-title": "Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients",
"author": "Qin",
"doi-asserted-by": "crossref",
"first-page": "e265",
"issue": "11",
"journal-title": "Transplantation",
"key": "10.1016/j.ijid.2022.03.015_bib0027",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial",
"author": "Spinner",
"doi-asserted-by": "crossref",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2022.03.015_bib0028",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1111/ajt.16579",
"article-title": "Spanish Society of Nephrology COVID-19 Group. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: Analysis of the Spanish Registry",
"author": "Villanego",
"doi-asserted-by": "crossref",
"first-page": "2573",
"issue": "7",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ijid.2022.03.015_bib0029",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(07)61602-X",
"article-title": "The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies",
"author": "von Elm",
"doi-asserted-by": "crossref",
"first-page": "1453",
"issue": "9596",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2022.03.015_bib0030",
"volume": "370",
"year": "2007"
},
{
"key": "10.1016/j.ijid.2022.03.015_bib0017",
"unstructured": "National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum. Accessed Nov 2021."
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971222001515"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "118"
}