Comparison of azvudine, molnupiravir, and nirmatrelvir/ritonavir in adult patients with mild-to-moderate COVID-19: a retrospective cohort study
et al., Scientific Reports, doi:10.1038/s41598-024-53862-y, Feb 2024
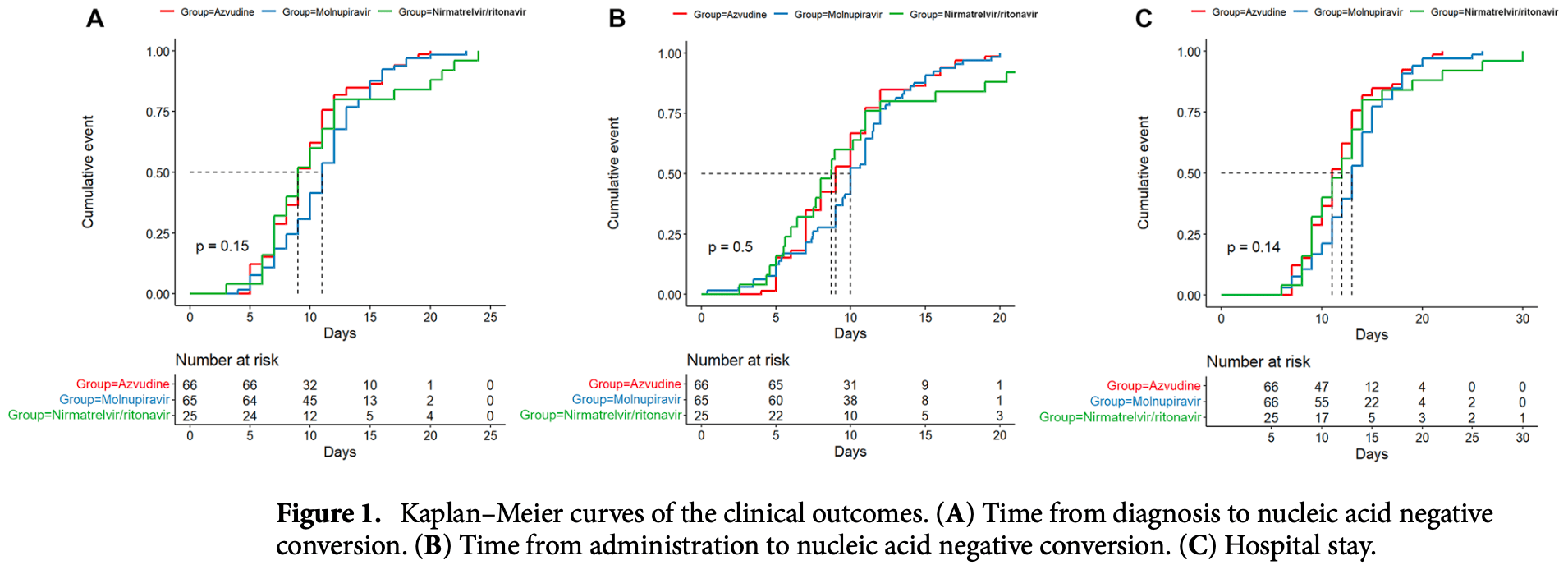
Retrospective 157 hospitalized mild-to-moderate COVID-19 patients showing no significant differences between azvudine, molnupiravir, and paxlovid for time to viral clearance and length of hospitalization.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Chen et al., 9 Feb 2024, retrospective, China, peer-reviewed, mean age 41.1, 5 authors, study period March 2022 - November 2022.
Contact: zhouzhiguo1217@163.com.
Comparison of azvudine, molnupiravir, and nirmatrelvir/ritonavir in adult patients with mild-to-moderate COVID-19: a retrospective cohort study
Scientific Reports, doi:10.1038/s41598-024-53862-y
This study aimed to explore the effectiveness and safety of azvudine, nirmatrelvir/ritonavir, and molnupiravir in adult patients with mild-to-moderate COVID-19. This retrospective cohort study included patients with mild-to-moderate COVID-19 (asymptomatic, mild, and common types) at the First Hospital of Changsha (Hunan Province, China) between March and November 2022. Eligible patients were classified into the azvudine, nirmatrelvir/ritonavir, or molnupiravir groups according to the antiviral agents they received. The outcomes were the times to nucleic acid negative conversion (NANC). This study included 157 patients treated with azvudine (n = 66), molnupiravir (n = 66), or nirmatrelvir/ritonavir (n = 25). There were no statistically significant differences in the time from diagnosis to NANC among the azvudine, molnupiravir, and nirmatrelvir/ritonavir groups [median, 9 (95% CI 9-11) vs. 11 (95% CI 10-12) vs. 9 (95% CI 8-12) days, P = 0.15], time from administration to NANC [median, 9 (95% CI 8-10) vs. 10 (95% CI 9.48-11) vs. 8.708 (95% CI 7.51-11) days, P = 0.50], or hospital stay [median, 11 (95% CI 11-13) vs. 13 (95% CI 12-14) vs. 12 (95% CI 10-14) days, P = 0.14], even after adjustment for sex, age, COVID-19 type, comorbidities, Ct level, time from diagnosis to antiviral treatment, and number of symptoms. The cumulative NANC rates in the azvudine, molnupiravir, and nirmatrelvir/ritonavir groups were 15.2%/12.3%/16.0% at day 5 (P = 0.858), 34.8%/21.5%/32.0% at day 7 (P = 0.226), 66.7%/52.3%/60.0% at 10 days (P = 0.246), and 86.4%/86.2%/80.0% at day 14 (P = 0.721). No serious adverse events were reported. Azvudine may be comparable to nirmatrelvir/ritonavir and molnupiravir in adult patients with mild-to-moderate COVID-19 regarding time to NANC, hospital stay, and AEs.
Author contributions Z.G.Z. and M.P.C. conceived and coordinated the study, designed, performed and analyzed the experiments, wrote the paper. D.X.J., J.X.R. and H.B.Z. carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Competing interests The authors declare no competing interests.
References
Anderson, Caubel, Rusnak, Investigators, Nirmatrelvir-ritonavir and viral load rebound in Covid-19, N. Engl. J. Med
Bernal, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N. Engl. J. Med
Butler, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial, Lancet
Casalini, Giacomelli, Antinori, Liver tests abnormalities with licensed antiviral drugs for COVID-19: A narrative review, Expert Opin. Drug Saf
Charness, Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment, N. Engl. J. Med
Consortium, Repurposed antiviral drugs for Covid-19-Interim WHO solidarity trial results, N. Engl. J. Med
Da Silva, Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front. Med
Dryden-Peterson, Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system: A population-based cohort study, Ann. Intern. Med
Gao, Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J. Infect
Gao, Liu, Li, Xu, Zhang et al., Molnupiravir for treatment of adults with mild or moderate COVID-19: A systematic review and meta-analysis of randomized controlled trials, Clin. Microbiol. Infect
Grandvuillemin, Rocher, Valnet-Rabier, Drici, Dautriche et al., Pharmacovigilance follow-up of patients in the context of the COVID-19 pandemic, Therapie
Hammond, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N. Engl. J. Med
Izzedine, Jhaveri, Perazella, COVID-19 therapeutic options for patients with kidney disease, Kidney Int
Kale, Shelke, Dagar, Anders, Gaikwad, How to use COVID-19 antiviral drugs in patients with chronic kidney disease, Front. Pharmacol
Khoo, Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): A randomised, placebo-controlled, double-blind, phase 2 trial, Lancet Infect. Dis
Koelle, Martin, Antia, Lopman, Dean, The changing epidemiology of SARS-CoV-2, Science
Lory, Safety profile of the lopinavir/ritonavir combination before and during the SARS-CoV-2 pandemic, Therapie
Niu, Ji, Zhao, Lei, Timing and magnitude of the second wave of the COVID-19 Omicron variant-189 Countries and Territories, November 2021 to, China CDC Wkly
Painter, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob. Agents Chemother
Parums, Editorial: Rebound COVID-19 and cessation of antiviral treatment for SARS-CoV-2 with paxlovid and molnupiravir, Med. Sci. Monit
Petrakis, Rafailidis, Trypsianis, Papazoglou, Panagopoulos, The antiviral effect of nirmatrelvir/ritonavir during COVID-19 pandemic real-world data, Viruses
Przekop, Gruszewska, Chrostek, Liver function in COVID-19 infection, World J. Hepatol
Qu, Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA, Cell Host Microbe
Ren, A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv. Sci
Ren, Wang, Gao, Zhou, Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance, World J. Clin. Cases
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J. Autoimmun
Rubin, From positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take paxlovid, JAMA
Sun, Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: A retrospective cohort study, ClinicalMedicine
Vassilopoulos, Mylonakis, In patients with COVID-19 at risk for severe disease, nirmatrelvir + ritonavir reduced hospitalization or death, Ann. Intern. Med
Vujcic, Outcomes of COVID-19 among patients with liver disease, World J. Gastroenterol
Wang, Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Cell
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): A review, JAMA
Yu, Liver injury in COVID-19: Clinical features and treatment management, Virol. J
Zhang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct. Target. Ther
Zhou, Clinical characteristics of older and younger patients infected with SARS-CoV-2, Aging
Zhou, Low-dose corticosteroid combined with immunoglobulin reverses deterioration in severe cases with COVID-19, Signal Transduct. Target. Ther
DOI record:
{
"DOI": "10.1038/s41598-024-53862-y",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-024-53862-y",
"abstract": "<jats:title>Abstract</jats:title><jats:p>This study aimed to explore the effectiveness and safety of azvudine, nirmatrelvir/ritonavir, and molnupiravir in adult patients with mild-to-moderate COVID-19. This retrospective cohort study included patients with mild-to-moderate COVID-19 (asymptomatic, mild, and common types) at the First Hospital of Changsha (Hunan Province, China) between March and November 2022. Eligible patients were classified into the azvudine, nirmatrelvir/ritonavir, or molnupiravir groups according to the antiviral agents they received. The outcomes were the times to nucleic acid negative conversion (NANC). This study included 157 patients treated with azvudine (n = 66), molnupiravir (n = 66), or nirmatrelvir/ritonavir (n = 25). There were no statistically significant differences in the time from diagnosis to NANC among the azvudine, molnupiravir, and nirmatrelvir/ritonavir groups [median, 9 (95% CI 9–11) vs. 11 (95% CI 10–12) vs. 9 (95% CI 8–12) days, <jats:italic>P</jats:italic> = 0.15], time from administration to NANC [median, 9 (95% CI 8–10) vs. 10 (95% CI 9.48–11) vs. 8.708 (95% CI 7.51–11) days, <jats:italic>P</jats:italic> = 0.50], or hospital stay [median, 11 (95% CI 11–13) vs. 13 (95% CI 12–14) vs. 12 (95% CI 10–14) days, <jats:italic>P</jats:italic> = 0.14], even after adjustment for sex, age, COVID-19 type, comorbidities, Ct level, time from diagnosis to antiviral treatment, and number of symptoms. The cumulative NANC rates in the azvudine, molnupiravir, and nirmatrelvir/ritonavir groups were 15.2%/12.3%/16.0% at day 5 (<jats:italic>P</jats:italic> = 0.858), 34.8%/21.5%/32.0% at day 7 (<jats:italic>P</jats:italic> = 0.226), 66.7%/52.3%/60.0% at 10 days (<jats:italic>P</jats:italic> = 0.246), and 86.4%/86.2%/80.0% at day 14 (<jats:italic>P</jats:italic> = 0.721). No serious adverse events were reported. Azvudine may be comparable to nirmatrelvir/ritonavir and molnupiravir in adult patients with mild-to-moderate COVID-19 regarding time to NANC, hospital stay, and AEs.</jats:p>",
"alternative-id": [
"53862"
],
"article-number": "3318",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "21 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "6 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 February 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Chen",
"given": "Mei-Ping",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Di-Xuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rang",
"given": "Jia-Xi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhuo",
"given": "Hai-Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Zhi-Guo",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T08:03:22Z",
"timestamp": 1707465802000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T08:06:08Z",
"timestamp": 1707465968000
},
"funder": [
{
"award": [
"2022SK2047",
"2020SK3014"
],
"name": "Key Research & Developmenta Program of Hunan Province"
},
{
"award": [
"kq2208448"
],
"name": "Natural Science Foundation of Changsha city"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
11
]
],
"date-time": "2024-02-11T12:15:44Z",
"timestamp": 1707653744037
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
2,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T00:00:00Z",
"timestamp": 1707436800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T00:00:00Z",
"timestamp": 1707436800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-024-53862-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-53862-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-53862-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
2,
9
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
9
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.12839",
"author": "WJ Wiersinga",
"doi-asserted-by": "publisher",
"first-page": "782",
"journal-title": "JAMA",
"key": "53862_CR1",
"unstructured": "Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): A review. JAMA 324, 782–793 (2020).",
"volume": "324",
"year": "2020"
},
{
"key": "53862_CR2",
"unstructured": "World Health Organization. COVID-19 Weekly Epidemiological Update. Edition 140 published 27 April 2023. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-2023. Accessed May 2, 2023. (World health Organization, Geneva, 2023)."
},
{
"DOI": "10.1126/science.abm4915",
"author": "K Koelle",
"doi-asserted-by": "publisher",
"first-page": "1116",
"journal-title": "Science",
"key": "53862_CR3",
"unstructured": "Koelle, K., Martin, M. A., Antia, R., Lopman, B. & Dean, N. E. The changing epidemiology of SARS-CoV-2. Science 375, 1116–1121 (2022).",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1016/j.jaut.2020.102433",
"author": "HA Rothan",
"doi-asserted-by": "publisher",
"journal-title": "J. Autoimmun.",
"key": "53862_CR4",
"unstructured": "Rothan, H. A. & Byrareddy, S. N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433 (2020).",
"volume": "109",
"year": "2020"
},
{
"key": "53862_CR5",
"unstructured": "China CDC. National Novel Coronavirus Infection Epidemic Situation. https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202304/t20230429_265709.html. Accessed June 14, 20232023."
},
{
"DOI": "10.1016/j.chom.2022.11.012",
"author": "P Qu",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Cell Host Microbe",
"key": "53862_CR6",
"unstructured": "Qu, P. et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA2752. Cell Host Microbe 31, 9–17 (2023).",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"author": "Q Wang",
"doi-asserted-by": "publisher",
"first-page": "279",
"journal-title": "Cell",
"key": "53862_CR7",
"unstructured": "Wang, Q. et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186, 279-286 e278 (2023).",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.12998/wjcc.v10.i1.1",
"author": "SY Ren",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "World J. Clin. Cases",
"key": "53862_CR8",
"unstructured": "Ren, S. Y., Wang, W. B., Gao, R. D. & Zhou, A. M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 10, 1–11 (2022).",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.46234/ccdcw2023.076",
"author": "B Niu",
"doi-asserted-by": "publisher",
"first-page": "397",
"journal-title": "China CDC Wkly.",
"key": "53862_CR9",
"unstructured": "Niu, B., Ji, S., Zhao, S. & Lei, H. Timing and magnitude of the second wave of the COVID-19 Omicron variant—189 Countries and Territories, November 2021 to February 2023. China CDC Wkly. 5, 397–401 (2023).",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "crossref",
"key": "53862_CR10",
"unstructured": "Consortium, W. H. O. S. T. et al. (2021) Repurposed antiviral drugs for Covid-19—Interim WHO solidarity trial results. N. Engl. J. Med. 384, 497–511."
},
{
"DOI": "10.7326/M22-2141",
"author": "S Dryden-Peterson",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Ann. Intern. Med.",
"key": "53862_CR11",
"unstructured": "Dryden-Peterson, S. et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system: A population-based cohort study. Ann. Intern. Med. 176, 77–84 (2023).",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.7326/J22-0038",
"author": "A Vassilopoulos",
"doi-asserted-by": "publisher",
"first-page": "JC63",
"journal-title": "Ann. Intern. Med.",
"key": "53862_CR12",
"unstructured": "Vassilopoulos, A. & Mylonakis, E. In patients with COVID-19 at risk for severe disease, nirmatrelvir + ritonavir reduced hospitalization or death. Ann. Intern. Med. 175, JC63 (2022).",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "53862_CR13",
"unstructured": "Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N. Engl. J. Med.",
"key": "53862_CR14",
"unstructured": "Jayk Bernal, A. et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00644-2",
"author": "SH Khoo",
"doi-asserted-by": "publisher",
"first-page": "183",
"journal-title": "Lancet Infect. Dis.",
"key": "53862_CR15",
"unstructured": "Khoo, S. H. et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): A randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect. Dis. 23, 183–195 (2023).",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Lancet",
"key": "53862_CR16",
"unstructured": "Butler, C. C. et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 401, 281–293 (2023).",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "J-L Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "53862_CR17",
"unstructured": "Zhang, J.-L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 6, 414 (2021).",
"volume": "6",
"year": "2021"
},
{
"author": "Z Ren",
"first-page": "e2001435",
"journal-title": "Adv. Sci. (Weinheim, Baden-Wurttemberg, Germany)",
"key": "53862_CR18",
"unstructured": "Ren, Z. et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinheim, Baden-Wurttemberg, Germany) 7, e2001435 (2020).",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"author": "RM da Silva",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"journal-title": "Front. Med.",
"key": "53862_CR19",
"unstructured": "da Silva, R. M. et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front. Med. 10, 1143485 (2023).",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"first-page": "e158",
"journal-title": "J. Infect.",
"key": "53862_CR20",
"unstructured": "Gao, Y. et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J. Infect. 86, e158–e160 (2023).",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.3390/v15040976",
"author": "V Petrakis",
"doi-asserted-by": "publisher",
"first-page": "976",
"journal-title": "Viruses",
"key": "53862_CR21",
"unstructured": "Petrakis, V., Rafailidis, P., Trypsianis, G., Papazoglou, D. & Panagopoulos, P. The antiviral effect of nirmatrelvir/ritonavir during COVID-19 pandemic real-world data. Viruses 15, 976 (2023).",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.2139/ssrn.4320807",
"doi-asserted-by": "crossref",
"key": "53862_CR22",
"unstructured": "Gao, Y., Liu, M., Li, Z., Xu, J., Zhang, J. & Tian, J. Molnupiravir for treatment of adults with mild or moderate COVID-19: A systematic review and meta-analysis of randomized controlled trials. Clin. Microbiol. Infect., (2023)."
},
{
"DOI": "10.18632/aging.103535",
"author": "Z Zhou",
"doi-asserted-by": "publisher",
"first-page": "11296",
"journal-title": "Aging (Albany NY)",
"key": "53862_CR23",
"unstructured": "Zhou, Z. et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging (Albany NY) 12, 11296–11305 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3748/wjg.v29.i5.815",
"author": "I Vujcic",
"doi-asserted-by": "publisher",
"first-page": "815",
"journal-title": "World J. Gastroenterol.",
"key": "53862_CR24",
"unstructured": "Vujcic, I. Outcomes of COVID-19 among patients with liver disease. World J. Gastroenterol. 29, 815–824 (2023).",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.4254/wjh.v13.i12.1909",
"author": "D Przekop",
"doi-asserted-by": "publisher",
"first-page": "1909",
"journal-title": "World J. Hepatol.",
"key": "53862_CR25",
"unstructured": "Przekop, D., Gruszewska, E. & Chrostek, L. Liver function in COVID-19 infection. World J. Hepatol. 13, 1909–1918 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s12985-021-01593-1",
"author": "D Yu",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Virol. J.",
"key": "53862_CR26",
"unstructured": "Yu, D. et al. Liver injury in COVID-19: Clinical features and treatment management. Virol. J. 18, 121 (2021).",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.therap.2023.01.004",
"doi-asserted-by": "crossref",
"key": "53862_CR27",
"unstructured": "Grandvuillemin, A., Rocher, F., Valnet-Rabier, M. B., Drici, M. D. & Dautriche, A. French Pharmacovigilance, N. Pharmacovigilance follow-up of patients in the context of the COVID-19 pandemic. Therapie, (2023)."
},
{
"DOI": "10.1016/j.therap.2022.10.066",
"author": "P Lory",
"doi-asserted-by": "publisher",
"first-page": "419",
"journal-title": "Therapie",
"key": "53862_CR28",
"unstructured": "Lory, P. et al. Safety profile of the lopinavir/ritonavir combination before and during the SARS-CoV-2 pandemic. Therapie 78, 419–425 (2022).",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.1080/14740338.2022.2160446",
"author": "G Casalini",
"doi-asserted-by": "publisher",
"first-page": "1483",
"journal-title": "Expert Opin. Drug Saf.",
"key": "53862_CR29",
"unstructured": "Casalini, G., Giacomelli, A. & Antinori, S. Liver tests abnormalities with licensed antiviral drugs for COVID-19: A narrative review. Expert Opin. Drug Saf. 21, 1483–1494 (2022).",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1128/AAC.02428-20",
"author": "WP Painter",
"doi-asserted-by": "publisher",
"first-page": "10-1128",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "53862_CR30",
"unstructured": "Painter, W. P. et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 65, 10–1128 (2021).",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"author": "A Kale",
"doi-asserted-by": "publisher",
"first-page": "1053814",
"journal-title": "Front. Pharmacol.",
"key": "53862_CR31",
"unstructured": "Kale, A., Shelke, V., Dagar, N., Anders, H. J. & Gaikwad, A. B. How to use COVID-19 antiviral drugs in patients with chronic kidney disease. Front. Pharmacol. 14, 1053814 (2023).",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.kint.2020.03.015",
"author": "H Izzedine",
"doi-asserted-by": "publisher",
"first-page": "1297",
"journal-title": "Kidney Int.",
"key": "53862_CR32",
"unstructured": "Izzedine, H., Jhaveri, K. D. & Perazella, M. A. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 97, 1297–1298 (2020).",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Y Sun",
"doi-asserted-by": "publisher",
"first-page": "e101981",
"journal-title": "ClinicalMedicine",
"key": "53862_CR33",
"unstructured": "Sun, Y. et al. Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: A retrospective cohort study. ClinicalMedicine 59, e101981 (2023).",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.12659/MSM.938532",
"author": "DV Parums",
"doi-asserted-by": "publisher",
"journal-title": "Med. Sci. Monit.",
"key": "53862_CR34",
"unstructured": "Parums, D. V. Editorial: Rebound COVID-19 and cessation of antiviral treatment for SARS-CoV-2 with paxlovid and molnupiravir. Med. Sci. Monit. 28, e938532 (2022).",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2205944",
"author": "AS Anderson",
"doi-asserted-by": "publisher",
"first-page": "1047",
"journal-title": "N. Engl. J. Med.",
"key": "53862_CR35",
"unstructured": "Anderson, A. S., Caubel, P., Rusnak, J. M. & Investigators, E.-H.T. Nirmatrelvir–ritonavir and viral load rebound in Covid-19. N. Engl. J. Med. 387, 1047–1049 (2022).",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2206449",
"author": "ME Charness",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "N. Engl. J. Med.",
"key": "53862_CR36",
"unstructured": "Charness, M. E. et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N. Engl. J. Med. 387, 1045–1047 (2022).",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.9925",
"author": "R Rubin",
"doi-asserted-by": "publisher",
"first-page": "2380",
"journal-title": "JAMA",
"key": "53862_CR37",
"unstructured": "Rubin, R. From positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take paxlovid. JAMA 327, 2380–2382 (2022).",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1038/s41392-020-00407-0",
"author": "ZG Zhou",
"doi-asserted-by": "publisher",
"first-page": "276",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "53862_CR38",
"unstructured": "Zhou, Z. G. et al. Low-dose corticosteroid combined with immunoglobulin reverses deterioration in severe cases with COVID-19. Signal Transduct. Target. Ther. 5, 276 (2020).",
"volume": "5",
"year": "2020"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-024-53862-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Comparison of azvudine, molnupiravir, and nirmatrelvir/ritonavir in adult patients with mild-to-moderate COVID-19: a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}
chen15