Metformin on the Presence of COVID-19 Symptoms Over 6 Months: The ACTIV-6 Randomized Clinical Trial
et al., medRxiv, doi:10.1101/2025.08.08.25333305, NCT04885530, Aug 2025
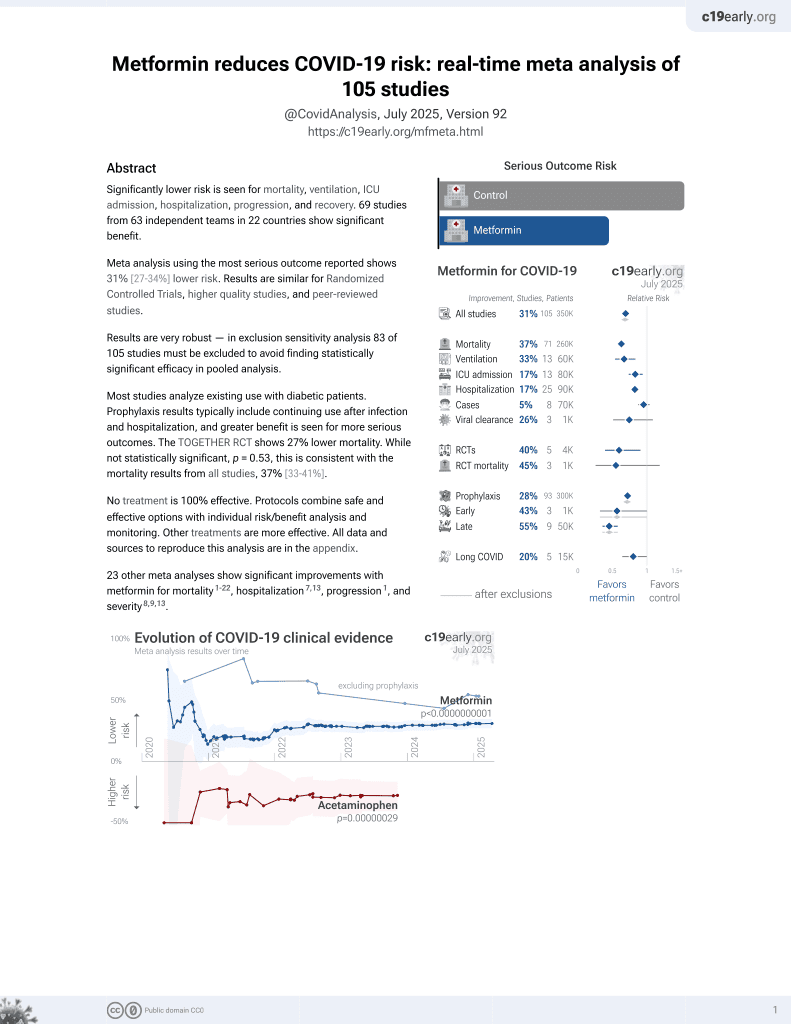
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
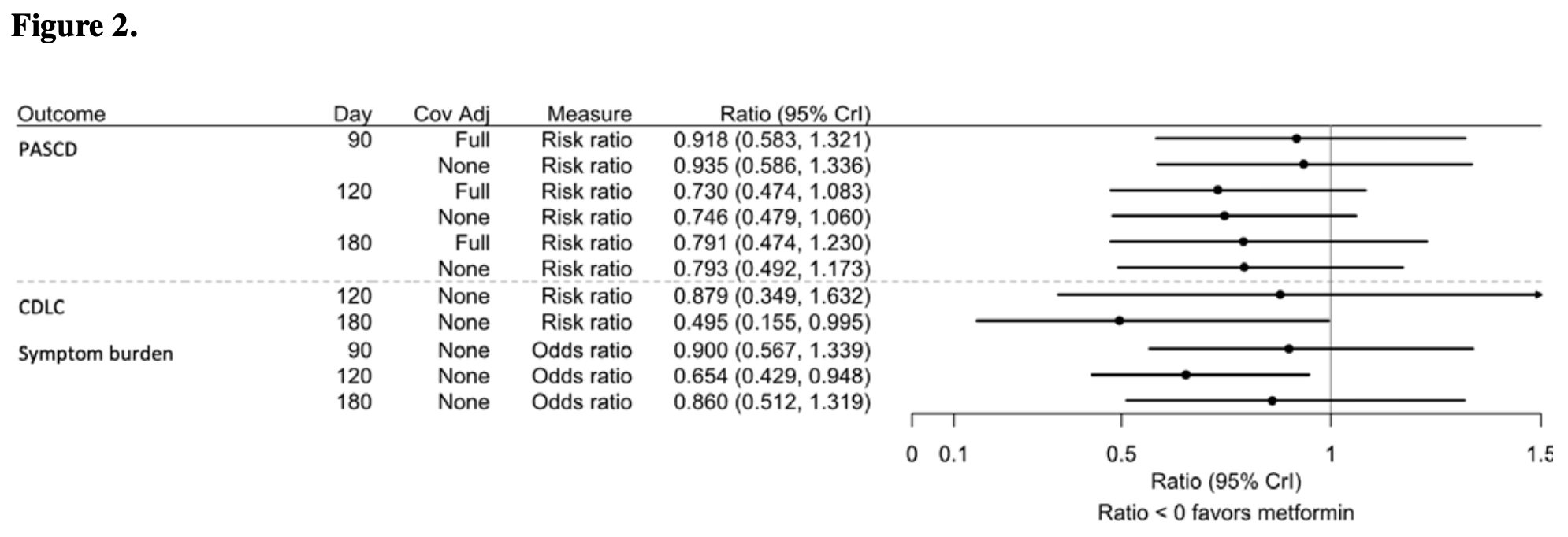
RCT 2,983 outpatients showing lower incidence of long COVID symptoms with metformin, without statistical significance. The primary endpoint of post-acute sequelae of COVID-19 or death at day 180 occurred in 2.3% of metformin patients vs 3.0% of placebo patients, with a posterior probability of efficacy of 0.83, which did not meet the prespecified threshold of 0.975 for declaring efficacy. Secondary outcomes favored metformin, including clinician diagnosis of long COVID at day 180 (0.56% vs 1.17%, posterior probability of efficacy 0.96). Results are reported with the main study entry1.
Bramante et al., 12 Aug 2025, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, median age 47.0, 27 authors, study period 19 September, 2023 - 1 May, 2024, trial NCT04885530 (history).
Contact: susanna.naggie@duke.edu.
Metformin on the Presence of COVID-19 Symptoms Over 6 Months: The ACTIV-6 Randomized Clinical Trial
doi:10.1101/2025.08.08.25333305
Background: The effect of metformin on preventing long-term COVID-19 symptoms among low-risk adults has not been studied. The objective of this study was to Assess metformin compared with placebo during acute SARS-CoV-2 infection on the presence of COVID-19 symptoms 180 days later.
Methods: The ACTIV-6 platform evaluated repurposed medications for mild to moderate COVID-19. Between September 19, 2023 and May 1, 2024, 2983 outpatient adults ≥30 years with confirmed SARS-CoV-2 infection and ≥2 COVID-19 symptoms for ≤7 days were included from 90 sites. Participants were randomized to metformin (titrated to 1500 mg daily) or placebo for 14 days. Post-acute sequelae of SARS-CoV-2 or death (PASCD) was ascertained by asking whether participants had symptoms they attributed to COVID-19 on day 180. Secondary outcomes included clinician diagnosis of long COVID. For the primary outcome, the single-sided threshold for efficacy was 0.975. Results: Among 2983 participants, the median age was 47 years (interquartile range [IQR] 38-57); 63% were female; 47% Hispanic/Latino; 83% reported ≥1 prior COVID-19 infections or SARS-CoV-2 vaccines. There were no deaths. Overall, 96 (3.2%) reported COVID-19 symptoms on day 90, 101 (3.4%) on day 120, and 79 (2.6%) on day 180. The covariate-adjusted risk of PASCD on day 180 was lower in the metformin group (-0.008; 95% credible interval [CrI] -0.022 to 0.006; posterior probability of efficacy [PPE] 0.83), compared with the placebo group with an adjusted risk ratio of 0.79 (95% CrI 0.474 to 1.230). The risk of clinician diagnosis of long COVID (secondary outcome) on day 180 was lower in the metformin group (-0.007; 95% CrI -0.015 to 0.001; PPE 0.96), with a relative risk of 0.495 (95% CrI 0.155 to 0.995).
Conclusions: The posterior probability of efficacy for metformin preventing the primary endpoint did not exceed the prespecified threshold of 0.975 for declaring efficacy. Secondary outcomes were numerically better with metformin.
Author Contributions Drs Naggie, Hernandez, and Lindsell had full access to all the blinded data in the study. Dr Stewart was provided curated study data and takes responsibility for the integrity of the data analysis. All authors contributed to the drafting and review of the manuscript and agreed to submit for publication.
References
Basting, Langat, Broedlow, SARS-CoV-2 infection is associated with intestinal permeability, systemic inflammation, and microbial dysbiosis in hospitalized COVID-19 patients, bioRxiv, doi:10.1101/2023.12.07.570670
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bramante, Beckman, Mehta, Favorable Antiviral Effect of Metformin on Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of Coronavirus Disease 2019, Clin Infect Dis, doi:10.1093/cid/ciae159
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadrupleblind, parallel-group, phase 3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00299-2
Bramante, Metformin reduces the risk of Long COVID or Death over 6 months in an Emulated Target Trial of Primarily Omicron-infected Adults without Diabetes or Prediabetes: a New-User, Active-Comparator Analysis Using the National COVID Cohort Collaborative (N3C) Electronic Health Record Database
Cao, Wang, Lu, Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2301425
Debari, Abbott, Adipose Tissue Fibrosis: Mechanisms, Models, and Importance, Int J Mol Sci, doi:10.3390/ijms21176030
Ely, Lisa, Harvey, Long Covid Defined, N Engl J Med, doi:10.1056/NEJMsb2408466
Fang, Ahrnsbrak, Rekito, Evidence Mounts That About 7% of US Adults Have Had Long COVID, JAMA, doi:10.1001/jama.2024.11370
Geng, Bonilla, Hedlin, Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial, JAMA Intern Med, doi:10.1001/jamainternmed.2024.2007
Giron, Peluso, Ding, Markers of fungal translocation are elevated during postacute sequelae of SARS-CoV-2 and induce NF-κB signaling, JCI Insight, doi:10.1172/jci.insight.160989
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Gutiérrez-Martínez, Rubio, Piedra-Quintero, mTORC1 Prevents Epithelial Damage During Inflammation and Inhibits Colitis-Associated Colorectal Cancer Development, Transl Oncol, doi:10.1016/j.tranon.2018.08.016
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hamrefors, Kahn, Holmqvist, Gut microbiota composition is altered in postural orthostatic tachycardia syndrome and post-acute COVID-19 syndrome, Sci Rep, doi:10.1038/s41598-024-53784-9
Harrell, None
Huang, Clinical, Institute, Lopes, Phd et al., the Duke Clinical Research Institute provided editorial support
Hunt, Efird, Redding, Medications Associated with Lower Mortality in a SARS-CoV-2 Positive Cohort of 26,508 Veterans, J Gen Intern Med, doi:10.1007/s11606-022-07701-3
Johnson, Sturmer, Prevalent Metformin Use in Adults with Diabetes and the Incidence of Long Covid: An EHR-based Cohort Study from the RECOVER Program
Kim, Hospital, John, Lantos, Silvey-Cason, Children's Mercy Hospital
Lally, Tsoukas, Halladay, Neill, Gravenstein et al., Metformin is Associated with Decreased 30-Day Mortality Among Nursing Home Residents Infected with SARS-CoV2, J Am Med Dir Assoc, doi:10.1016/j.jamda.2020.10.031
Levy, Chilunda, Davis, Reduced Likelihood of Hospitalization with the JN.1 or HV.1 SARS-CoV-2 Variants Compared to the EG.5 Variant, J Infect Dis, doi:10.1093/infdis/jiae364
Lewnard, Mahale, Malden, Immune escape and attenuated severity associated with the SARS-CoV-2 BA.2.86/JN.1 lineage, Nat Commun, doi:10.1038/s41467-024-52668-w
Liu, Mak, Su, Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome, Gut, doi:10.1136/gutjnl-2021-325989
Lukito, Pranata, Henrina, Lim, Lawrensia et al., The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and metaanalysis, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.11.006
Ma, Castro, Lambrou, Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron XBB and JN.1 Lineages -United States, May 2023-September 2024, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7342a1
Mateu, Tebe, Loste, Determinants of the onset and prognosis of the post-COVID-19 condition: a 2-year prospective observational cohort study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2023.100724
Naggie, Boulware, Lindsell, Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590
Nistala, Raja, Pulakat, mTORC1 inhibitors rapamycin and metformin affect cardiovascular markers differentially in ZDF rats, Can J Physiol Pharmacol, doi:10.1139/cjpp-2016-0567%M28177677
Parthasarathy, Tandel, Siddiqui, Harshan, Metformin suppresses SARS-CoV-2 in cell culture, Virus Res, doi:10.1016/j.virusres.2022.199010
Placeholder, CB will try to get the COVID-OUT stool samples analyzed
Planas, Pagliuzza, Ponte, LILAC pilot study: Effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy, EBioMedicine. Mar, doi:10.1016/j.ebiom.2021.103270
Qu, Evans, Faraone, Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, Cell Host Microbe, doi:10.1016/j.chom.2022.11.012
Schaller, Sharma, Dupee, Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses, JCI Insight, doi:10.1172/jci.insight.148003
Schulz, Altman, Moher, Group, CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials, Trials, doi:10.1186/1745-6215-11-32
Soff, Yoo, Bramante, Association of glycemic control with Long COVID in patients with type 2 diabetes: findings from the National COVID Cohort Collaborative (N3C), BMJ Open Diabetes Res Care, doi:10.1136/bmjdrc-2024-004536
Song, Huang, Xu, Zhou, Zhang, The Effect of Antihyperglycemic Medications on COVID-19: A Meta-analysis and Systematic Review from Observational Studies, Ther Innov Regul Sci, doi:10.1007/s43441-024-00633-6
Team, RStan: the R interface to Stan
Telesford, Mcgough, Tevis, Cotter, How has the burden of chronic diseases in the U.S. and peer nations changed over time?
Usman, Bliden, Cho, Metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes, J Thromb Thrombolysis, doi:10.1007/s11239-022-02631-7
Ventura-López, Cervantes-Luevano, Aguirre-Sánchez, Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113223
Wang, Mellis, Ho, Recurrent SARS-CoV-2 spike mutations confer growth advantages to select JN.1 sublineages, Emerg Microbes Infect, doi:10.1080/22221751.2024.2402880
Wong, Devason, Umana, Serotonin reduction in post-acute sequelae of viral infection, Cell, doi:10.1016/j.cell.2023.09.013
Xian, Liu, Nilsson, Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation, Immunity, doi:10.1016/j.immuni.2021.05.0041139(38.2
Xie, Zhao, Shi, Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer, J Clin Invest, doi:10.1172/JCI133264
Zambalde, Dias, Maktura, Increased mTOR Signaling and Impaired Autophagic Flux Are Hallmarks of SARS-CoV-2 Infection, Curr Issues Mol Biol, doi:10.3390/cimb45010023
Zhang, Lau, Liu, Su, Chan et al., Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications, Nat Rev Gastroenterol Hepatol, doi:10.1038/s41575-022-00698-4
DOI record:
{
"DOI": "10.1101/2025.08.08.25333305",
"URL": "http://dx.doi.org/10.1101/2025.08.08.25333305",
"abstract": "<jats:p>Background: The effect of metformin on preventing long-term COVID-19 symptoms among low-risk adults has not been studied. The objective of this study was to Assess metformin compared with placebo during acute SARS-CoV-2 infection on the presence of COVID-19 symptoms 180 days later. \nMethods: The ACTIV-6 platform evaluated repurposed medications for mild to moderate COVID-19. Between September 19, 2023 and May 1, 2024, 2983 outpatient adults >=30 years with confirmed SARS-CoV-2 infection and >=2 COVID-19 symptoms for <=7 days were included from 90 sites. Participants were randomized to metformin (titrated to 1500 mg daily) or placebo for 14 days. Post-acute sequelae of SARS-CoV-2 or death (PASCD) was ascertained by asking whether participants had symptoms they attributed to COVID-19 on day 180. Secondary outcomes included clinician diagnosis of long COVID. For the primary outcome, the single-sided threshold for efficacy was 0.975.\nResults: Among 2983 participants, the median age was 47 years (interquartile range [IQR] 38-57); 63% were female; 47% Hispanic/Latino; 83% reported >=1 prior COVID-19 infections or SARS-CoV-2 vaccines. There were no deaths. Overall, 96 (3.2%) reported COVID-19 symptoms on day 90, 101 (3.4%) on day 120, and 79 (2.6%) on day 180. The covariate-adjusted risk of PASCD on day 180 was lower in the metformin group (-0.008; 95% credible interval [CrI] -0.022 to 0.006; posterior probability of efficacy [PPE] 0.83), compared with the placebo group with an adjusted risk ratio of 0.79 (95% CrI 0.474 to 1.230). The risk of clinician diagnosis of long COVID (secondary outcome) on day 180 was lower in the metformin group (-0.007; 95% CrI -0.015 to 0.001; PPE 0.96), with a relative risk of 0.495 (95% CrI 0.155 to 0.995). \nConclusions: The posterior probability of efficacy for metformin preventing the primary endpoint did not exceed the prespecified threshold of 0.975 for declaring efficacy. Secondary outcomes were numerically better with metformin. \nTrial Registration: ClinicalTrials.gov (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04885530\">NCT04885530</jats:ext-link>).</jats:p>",
"accepted": {
"date-parts": [
[
2025,
8,
12
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0001-5858-2080",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bramante",
"given": "Carolyn",
"sequence": "first"
},
{
"affiliation": [],
"family": "Stewart",
"given": "Thomas G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boulware",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCarthy",
"given": "Matthew W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gao",
"given": "Yue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rothman",
"given": "Russell L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourad",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thicklin",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia del Sol",
"given": "Idania T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Nirav S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehta",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quintero Cardona",
"given": "Orlando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scott",
"given": "Jake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castro",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jayaweera",
"given": "Dushyantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sulkowski",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McTigue",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Felker",
"given": "G. Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collins",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dunsmore",
"given": "Sarah E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adam",
"given": "Stacey J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindsell",
"given": "Christopher J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernandez",
"given": "Adrian F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naggie",
"given": "Susanna",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T01:40:11Z",
"timestamp": 1755049211000
},
"deposited": {
"date-parts": [
[
2025,
8,
13
]
],
"date-time": "2025-08-13T01:40:12Z",
"timestamp": 1755049212000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2025,
8,
15
]
],
"date-time": "2025-08-15T02:58:18Z",
"timestamp": 1755226698014,
"version": "3.43.0"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
8,
12
]
]
},
"license": [
{
"URL": "https://www.medrxiv.org/about/FAQ#license",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
12
]
],
"date-time": "2025-08-12T00:00:00Z",
"timestamp": 1754956800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.08.08.25333305",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
8,
12
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
8,
12
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2025.08.08.25333305"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Metformin on the Presence of COVID-19 Symptoms Over 6 Months: The ACTIV-6 Randomized Clinical Trial",
"type": "posted-content"
}