Metformin and Time to Sustained Recovery in Adults With COVID-19
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2025.2570, ACTIV-6, NCT04885530, Jan 2025 (preprint)
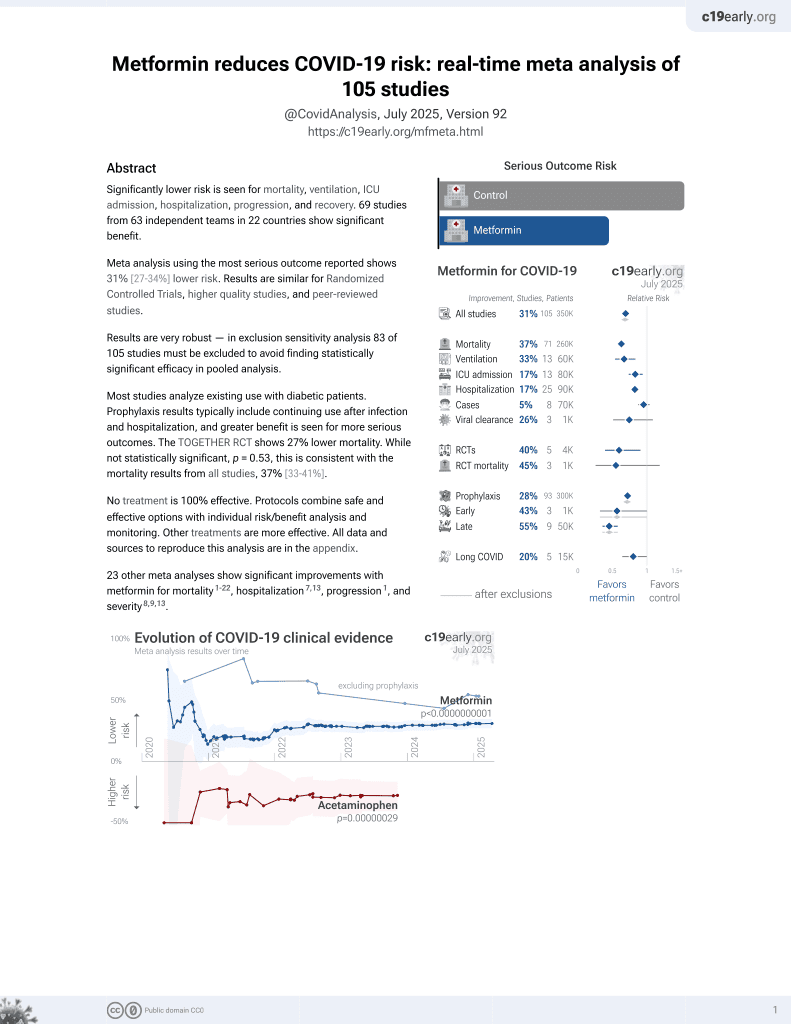
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 2,991 low-risk outpatients including patients up to 12 days from symptoms (despite the protocol being up to 7 days), showing no significant differences with metformin. Remote trial with 2 days shipping delay. Median days to symptom resolution was 9 days vs. 10 days for placebo, without statistical significance. There was a median 5 day delay for drug receipt (treatment delay is unspecified but may be greater).
eFigure 5 shows HR 0.40 [0.28-0.58], p = 0.000001, for patients that had recovered by the time of drug receipt and had no symptoms on that day. This result suggests a major confounding factor or flaw in the study leading to very unreliable results and a strong bias towards the placebo group. A likely possible cause is the inclusion of metformin side effects as COVID-19 symptoms. Authors include many side effects of metformin as COVID-19 symptoms: diarrhea: a common side effect of metformin, especially in the initial days of treatment; nausea: frequently reported with metformin use, vomiting: less common but a documented side effect of metformin; fatigue: metformin may cause mild fatigue or malaise in some individuals, and headache: rare but also a possible side effect of metformin. Authors do a sensitivity analysis using day 1 vs. baseline symptoms, however they do not provide details of this analysis. The text suggests authors only used diarrhea, and the day 1 focus would also result in only partial correction.
Note that it would be simple for authors to perform an analysis focusing on more COVID-19 specific and/or serious symptoms. Symptoms included: overall symptom burden, fatigue, dyspnea, fever, cough, nausea, vomiting, diarrhea, body aches, sore throat, headache, chills, nasal symptoms, new loss of sense of taste or smell, and other COVID-related symptoms.
Only 7 patients were hospitalized and no information is given on their severity. For other issues with this trial see Naggie et al., where many of the issues also apply to this arm of the trial.
Trial designs favoring placebo/no effect were likely done to minimize efficacy of an earlier treatment in the trial - for example the wide inclusion of non-COVID-19 specific symptoms, inclusion of typical side-effect symptoms, use of the last of 3 days instead of the first of 3 days for sustained recovery, very slow shipping, and inclusion of patients up to 12 days from onset.
Patients with symptoms ≤7 days from onset were eligible, however eFigure 1 shows there were patients with up to 12 days from symptoms to drug receipt, suggesting up to 5 days shipping delay. Trial operation is not logical for an acute condition like COVID-19. Table 1 shows 48 hours delay between enrollment and receipt of medication (treatment time is not reported and may be even later - patients may not be at the location at delivery time). It is unclear why authors would not use overnight shipping as a worst case, widely available for the study population, for <24 hours delivery (other than designing the trial to favor finding no effect).
The study period was September 2023 - May 2024, by which time SARS-CoV-2 variants resulted in signficantly fewer serious outcomes, reducing the potential for a treatment to show a significant affect on serious outcomes.
There is an extensive list of major conflicts of interest reported (any many unreported).
ACTIV trial authors have reported a number of issues that may affect
the reliability of the results in ACTIV trials including
participant fraud2,
biased participant demographics3,
resource issues that may have led to protocol deviations3,
differences in trial design including inconsistent inclusion/exclusion
criteria3,
participant self-selection bias2,3,
underrepresentation of older patients due to web-based recruitment3,
changes in treatment and public health policies during trials3,
treatment delay determination from shipping logs and delivery that may not be directly
to the patient2,
variable placebo responses (e.g., oral vs. inhaled)4,
logistical challenges maintaining blinding4,
errors from complex data collection systems4,
unplanned design changes including endpoint changes4, and
inconsistent SoC across trial sites and time periods4.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments5.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
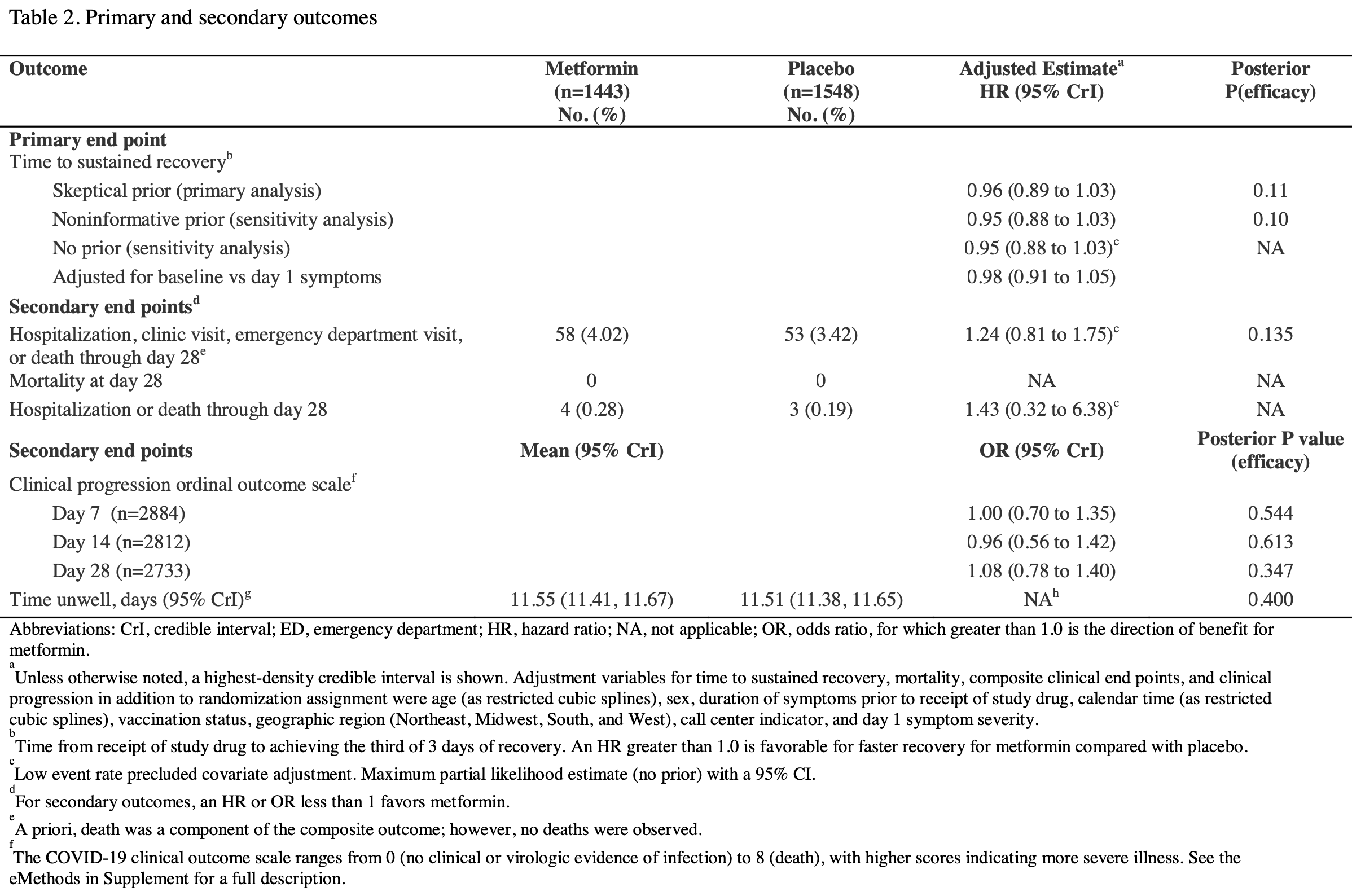
risk of hospitalization, 43.0% higher, HR 1.43, p = 0.65, treatment 4 of 1,443 (0.3%), control 3 of 1,548 (0.2%), day 28.
|
|
hosp. or ED visit, 20.0% lower, HR 0.80, p = 0.52, treatment 15 of 1,443 (1.0%), control 20 of 1,548 (1.3%), NNT 396, day 28.
|
|
risk of progression, 25.0% higher, HR 1.25, p = 0.26, treatment 54 of 1,443 (3.7%), control 59 of 1,548 (3.8%), adjusted per study, hospitalization, clinic visit, ER visit, or death, day 28.
|
|
risk of progression, 8.0% higher, HR 1.08, p = 0.63, treatment 1,443, control 1,548, adjusted per study, ordinal scale, day 28.
|
|
risk of progression, 4.0% lower, HR 0.96, p = 0.87, treatment 1,443, control 1,548, adjusted per study, ordinal scale, day 14.
|
|
risk of progression, 1.0% lower, HR 0.99, p = 0.96, treatment 1,443, control 1,548, adjusted per study, ordinal scale, day 7.
|
|
risk of no recovery, 4.2% higher, HR 1.04, p = 0.28, treatment 58 of 1,443 (4.0%), control 53 of 1,548 (3.4%), adjusted per study, inverted to make HR<1 favor treatment, skeptical prior.
|
|
risk of no recovery, 10.0% lower, RR 0.90, p = 0.006, treatment mean 9.0 (±9.69) n=1,443, control mean 10.0 (±10.0) n=1,548, relative median days to sustained recovery, last of three days.
|
|
risk of no recovery, 12.5% lower, RR 0.88, p = 0.006, treatment mean 7.0 (±9.69) n=1,443, control mean 8.0 (±10.0) n=1,548, relative median days to sustained recovery, first of three days.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Naggie et al., Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590.
2.
Lindsell et al., ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19, Journal of Clinical and Translational Science, doi:10.1017/cts.2023.644.
3.
Wohl et al., Engaging communities in therapeutics clinical research during pandemics: Experiences and lessons from the ACTIV COVID-19 therapeutics research initiative, Journal of Clinical and Translational Science, doi:10.1017/cts.2024.561.
Bramante et al., 14 Jan 2025, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 47.0, 28 authors, study period 19 September, 2023 - 1 May, 2024, average treatment delay 5.0 days, trial NCT04885530 (history) (ACTIV-6).
Contact: susanna.naggie@duke.edu.
Metformin on Time to Sustained Recovery in Adults with COVID-19: The ACTIV-6 Randomized Clinical Trial
doi:10.1101/2025.01.13.25320485
Importance: The effect of metformin on reducing symptom duration among outpatient adults with coronavirus disease 2019 (COVID-19) has not been studied. Objective: Assess metformin compared with placebo for symptom resolution during acute infection with SARS-CoV-2.
Design , Setting, and Participants: The ACTIV-6 platform evaluated repurposed medications for mild to moderate COVID-19. Between September 19, 2023, and May 1, 2024, 2991 participants age ≥30 years with confirmed SARS-CoV-2 infection and ≥2 COVID-19 symptoms for ≤7 days, were included at 90 US sites. Interventions: Participants were randomized to receive metformin (titrated to 1500 mg daily) or placebo for 14 days. Main Outcomes and Measures: The primary outcome was time to sustained recovery (3 consecutive days without COVID-19 symptoms) within 28 days of receiving study drug. Secondary outcomes included time to hospitalization or death; time to healthcare utilization (clinic visit, emergency department visit, hospitalization, or death). Safety events of special interest were hypoglycemia and lactic acidosis. Results: Among 2991 participants who were randomized and received study drug, the median age was 47 years (IQR 38-58); 63.4% were female, 46.5% identified as Hispanic/Latino, and 68.3% reported ≥2 doses of a SARS-CoV-2 vaccine. Among 1443 participants who received metformin and 1548 who received placebo, differences in time to sustained recovery were not observed (adjusted hazard ratio [aHR] 0.96; 95% credible interval [CrI] 0.89-1.03; P(efficacy)=0.11). For participants enrolled during current variants, the aHR was 1.19 (95% CrI 1.05-1.34). The median time to sustained recovery was 9 days (95% confidence interval [CI] 9-10) for metformin and 10 days (95% CI 9-10) for placebo. No deaths were reported; 111 participants reported healthcare utilization: 58 in the metformin group and 53 in the placebo group (HR 1.24; 95% CrI 0.81-1.75; P(efficacy)=0.135). Seven participants who received metformin and 3 who received placebo experienced a serious adverse event over 180 days. Five participants in each group reported having hypoglycemia.
Author Contributions Drs Naggie, Hernandez, and Lindsell had full access to all the blinded data in the study. Dr Stewart was provided curated study data and takes responsibility for the integrity of the data analysis. All authors contributed to the drafting and review of the manuscript and agreed to submit for publication.
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bramante, Beckman, Mehta, Favorable Antiviral Effect of Metformin on Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of Coronavirus Disease 2019, Clin Infect Dis, doi:10.1093/cid/ciae159
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadrupleblind, parallel-group, phase 3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00299-2
Bramante, Huling, Tignanelli, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Bramante, Metformin reduces the risk of Long COVID or Death over 6 months in an Emulated Target Trial of Primarily Omicron-infected Adults without Diabetes or Prediabetes: a New-User, Active-Comparator Analysis Using the National COVID Cohort Collaborative (N3C) Electronic Health Record Database
Campo, García-Valdecasas, Gil-Gómez, Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy, PLoS One, doi:10.1371/journal.pone.0191805
Cao, Wang, Lu, Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2301425
Chan, Casiraghi, Laraway, Metformin is associated with reduced COVID-19 severity in patients with prediabetes, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2022.110157
Crouse, Grimes, Li, Might, Ovalle et al., Metformin Use Is Associated With Reduced Mortality in a Diverse Population With COVID-19 and Diabetes, Front Endocrinol, doi:10.3389/fendo.2020.600439
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2118542
Harris, Taylor, Minor, The REDCap consortium: Building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:10.1016/j.jbi.2008.08.010
Holmberg, Andersen, Ajusting for Covariates in Randomized Clinical Trials for Drugs and Biological Products, JAMA, doi:10.1001/jama.2022.21506
Hunt, Efird, Redding, Medications Associated with Lower Mortality in a SARS-CoV-2 Positive Cohort of 26,508 Veterans, Journal of General Internal Medicine, doi:10.1007/s11606-022-07701-3
Ibrahim, Lowe, Bramante, Metformin and Covid-19: Focused Review of Mechanisms and Current Literature Suggesting Benefit, Front Endocrinol, doi:10.3389/fendo.2021.587801
Karam, Morris, Bramante, mTOR inhibition in COVID-19: A commentary and review of efficacy in RNA viruses, Journal of Medical Virology, doi:10.1002/jmv.26728
Karim, Lo, Einav, Preparing for the next viral threat with broad-spectrum antivirals, J Clin Invest, doi:10.1172/jci170236
Kow, Hasan, Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis, J Med Virol, doi:10.1002/jmv.26498
Lee, Boulware, Is there room for metformin at COVID-19's dinner table? Updated Analysis of Clinical Trials, Clin Infect Dis, doi:10.1093/cid/ciae28429
Levy, Chilunda, Davis, Reduced Likelihood of Hospitalization with the JN.1 or HV.1 SARS-CoV-2 Variants Compared to the EG.5 Variant, J Infect Dis, doi:10.1093/infdis/jiae364
Lewnard, Mahale, Malden, Immune escape and attenuated severity associated with the SARS-CoV-2 BA.2.86/JN.1 lineage, Nat Commun, doi:10.1038/s41467-024-52668-w
Ma, Castro, Lambrou, Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron XBB and JN.1 Lineages -United States, May 2023-September 2024, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7342a1
Naggie, Boulware, Lindsell, Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590
Nakashima, Takeuchi, Chihara, Hotta, Sada, Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways, Microbiol Immunol, doi:10.1111/j.1348-0421.2011.00382.x
Parthasarathy, Tandel, Siddiqui, Harshan, Metformin suppresses SARS-CoV-2 in cell culture, Virus Res, doi:10.1016/j.virusres.2022.199010
Pocock, Stone, The Primary Outcome Fails -What Next?, New England Journal of Medicine, doi:10.1056/NEJMra1510064
Qiu, Hubbard, Gutiérrez, Estimating the effect of realistic improvements of metformin adherence on COVID-19 mortality using targeted machine learning, Glob Epidemiol, doi:10.1016/j.gloepi.2024.100142
Qu, Evans, Faraone, Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, Cell Host Microbe, doi:10.1016/j.chom.2022.11.012
Rohde, French, Stewart, Harrell, Bayesian transition models for ordinal longitudinal outcomes, Stat Med, doi:10.1002/sim.10133
Schaller, Sharma, Dupee, Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses, JCI Insight, doi:10.1172/jci.insight.148003
Schulz, Altman, Moher, Group, CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials, Trials, doi:10.1186/1745-6215-11-32
Shinohara, Imajo, Yoneda, Unfolded protein response pathways regulate Hepatitis C virus replication via modulation of autophagy, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2013.01.103
Song, Huang, Xu, Zhou, Zhang, The Effect of Antihyperglycemic Medications on COVID-19: A Meta-analysis and Systematic Review from Observational Studies, Ther Innov Regul Sci, doi:10.1007/s43441-024-00633-6
Soto-Acosta, Bautista-Carbajal, Cervantes-Salazar, Angel-Ambrocio, Angel, DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target, PLoS Pathog, doi:10.1371/journal.ppat.1006257
Tsai, Chang, Sun, Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase, Oncotarget, doi:10.18632/oncotarget.20248
Usman, Bliden, Cho, Metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes, J Thromb Thrombolysis, doi:10.1007/s11239-022-02631-7
Ventura-López, Cervantes-Luevano, Aguirre-Sánchez, Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113223
Wang, Mellis, Ho, Recurrent SARS-CoV-2 spike mutations confer growth advantages to select JN.1 sublineages, Emerg Microbes Infect, doi:10.1080/22221751.2024.2402880
Xian, Liu, Nilsson, Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation, Immunity, doi:10.1016/j.immuni.2021.05.004
Zhang, Feng, Luo, Metformin Hydrochloride Significantly Inhibits Rotavirus Infection in Caco2 Cell Line, Intestinal Organoids, and Mice, Pharmaceuticals, doi:10.3390/ph16091279
DOI record:
{
"DOI": "10.1001/jamainternmed.2025.2570",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2025.2570",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>The effect of metformin on reducing symptom duration among outpatient adults with COVID-19 has not been studied.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess metformin compared with placebo for symptom resolution during acute infection with SARS-CoV-2.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>The Accelerating COVID-19 Therapeutic Interventions and Vaccines platform evaluated repurposed medications for mild to moderate COVID-19. Between September 19, 2023, and May 1, 2024, participants 30 years or older with confirmed SARS-CoV-2 infection and 2 or more COVID-19 symptoms for 7 days or less were included at 90 US sites.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants were randomized to receive metformin (titrated to 1500 mg, daily) or placebo for 14 days.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was time to sustained recovery (3 consecutive days without COVID-19 symptoms) within 28 days of receiving the study drug. Secondary outcomes included time to clinic visit, emergency department (ED) visit, hospitalization, or death. Safety events of interest were hypoglycemia and lactic acidosis.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 2991 participants who were randomized and received study drug, the median age was 47 (IQR, 38-58) years; 1895 (63.4%) were female, 25 (0.8%) were American Indian of Alaska Native, 77 (2.6%) were Asian, 350 (11.7%) were Black, African American, or African, 1392 (46.5%) identified as Hispanic or Latino, 8 (0.3%) were Native Hawaiian or other Pacific Islander, 2395 (80.1%) were White, and 2044 (68.3%) reported 2 or more doses of a SARS-CoV-2 vaccine. Among 1443 (48.2%) participants who received metformin and 1548 (51.8%) who received placebo, differences in time to sustained recovery were not observed (adjusted hazard ratio, 0.96; 95% credible interval [CrI], 0.89-1.03; <jats:italic>P for efficacy</jats:italic> = .11). The median time to sustained recovery was 9 days (95% CI, 9-10) for metformin and 10 days (95% CI, 9-10) for placebo. No deaths were reported; 103 participants reported clinic visits, ED visits, or hospitalization: 54 in the metformin group and 49 in the placebo group (hazard ratio, 1.25; 95% CrI, 0.82-1.78; <jats:italic>P for efficacy</jats:italic> = .13). Overall, 35 (1.2%) reported ED visits or hospitalization (1.1% in the metformin and 1.3% in the placebo group). Seven participants who received metformin and 3 who received placebo experienced a serious adverse event over 180 days. There were 4 episodes of participant-reported hypoglycemia in the placebo group and 2 in the metformin group.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In this randomized clinical trial, metformin was not shown to shorten the time to symptom resolution in low-risk adults with COVID-19. The median days to symptom resolution was numerically but not significantly lower for metformin. Safety was not a limitation in the study population.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/study/NCT04885530\">NCT04885530</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Medicine, University of Minnesota Medical School, Minneapolis"
}
],
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Data Science, University of Virginia, Charlottesville"
}
],
"family": "Stewart",
"given": "Thomas G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Minnesota Medical School, Minneapolis"
}
],
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Weill Cornell Medicine, New York, New York"
}
],
"family": "McCarthy",
"given": "Matthew W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Gao",
"given": "Yue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Rothman",
"given": "Russell L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Duke University School of Medicine, Durham, North Carolina"
},
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Mourad",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stakeholder Advisory Committee, Pittsburgh, Pennsylvania"
}
],
"family": "Thicklin",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jadestone Clinical Research LLC, Silver Spring, Maryland"
}
],
"family": "Cohen",
"given": "Jonathan B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "L&A Morales Healthcare Inc, Miami, Florida"
}
],
"family": "Garcia del Sol",
"given": "Idania T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innovation Clinical Trials Inc, Miami, Florida"
}
],
"family": "Ruiz-Unger",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Endeavor Health, Evanston, Illinois"
}
],
"family": "Shah",
"given": "Nirav S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GCF of Southeastern Michigan, PC, Detroit"
}
],
"family": "Mehta",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Cardona",
"given": "Orlando Quintero",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Scott",
"given": "Jake",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Colorado School of Medicine, Aurora"
}
],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Critical Care and Sleep Medicine, University of Kansas Medical Center, Kansas City"
}
],
"family": "Castro",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Miller School of Medicine, University of Miami, Miami, Florida"
}
],
"family": "Jayaweera",
"given": "Dushyantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Sulkowski",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania"
}
],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania"
}
],
"family": "McTigue",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Duke University School of Medicine, Durham, North Carolina"
},
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Felker",
"given": "G. Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt University Medical Center, Nashville, Tennessee"
},
{
"name": "Veterans Affairs Tennessee Valley Healthcare System, Geriatric Research, Education and Clinical Center, Nashville, Tennessee"
}
],
"family": "Collins",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Dunsmore",
"given": "Sarah E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Foundation for the National Institutes of Health, Bethesda, Maryland"
}
],
"family": "Adam",
"given": "Stacey J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Hernandez",
"given": "Adrian F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Naggie",
"given": "Susanna",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "DeLong",
"given": "Allison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wilder",
"given": "Rhonda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Hanna",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Fraser",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ward",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Weaver",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "McAdams",
"given": "M. Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Korzekwinski",
"given": "Kayla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Oyelakin",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Dockery",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Adkins",
"given": "Rodney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Crow",
"given": "Mathew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Light",
"given": "Shanee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Morris",
"given": "Renee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nowell",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wells",
"given": "Kadie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Herbert",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Stone",
"given": "Allegra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Heavlin",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Brown",
"given": "Linley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Brunson",
"given": "Shelley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Harding",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Harrington",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Beauchaine",
"given": "Meaghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lindblom",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Burns",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mourad",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Hamm",
"given": "Megan E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Phillips",
"given": "Kirk T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vasey",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Edwards",
"given": "Talethia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nelson",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Merritt",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nguyen",
"given": "Jeannie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Denson",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Arnold",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Aamodt",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Clark",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Collins",
"given": "Jess",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Collins",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Dixon",
"given": "Sheri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gao",
"given": "Yue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Graves",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Grindstaff",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Harrell",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lai",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Liao",
"given": "Vicky",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lopez",
"given": "Itzel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Manis",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mankowski",
"given": "Kalley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Marlin",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Merkel",
"given": "Alyssa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nwosu",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Obregon",
"given": "Savannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Orozco",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Prato",
"given": "Nelson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rohde",
"given": "Max",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Shirey-Rice",
"given": "Jana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vermillion",
"given": "Krista",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Smith",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Tan",
"given": "Hsi-nien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vance",
"given": "Meghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Weir",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bianchi",
"given": "Ray",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Premas",
"given": "Jen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gupta",
"given": "Madhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Karawan",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lima",
"given": "Santia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ziomek",
"given": "Carey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Arena",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "DeAlmeida",
"given": "Sonaly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Malik",
"given": "Anuj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bryce",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Swint",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Tiffany",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Tanner",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Sahelian",
"given": "Allegra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "George-Adebayo",
"given": "Constance",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Adebayo",
"given": "Adeolu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ronan",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Woods",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gallegos",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Flys",
"given": "Tamara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Sloan",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Olofintuyi",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Samraj",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Samraj",
"given": "Jackelyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Averett",
"given": "Amaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Slandzicki",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wallan",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vogel",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Munoz",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Kavtaradze",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Watson",
"given": "Casandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Singleton",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Sevier",
"given": "Marcus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rivon",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rao",
"given": "Sohail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cantu",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Krishna",
"given": "Arvind",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Daugherty",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Kerr",
"given": "Brandi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Evans",
"given": "Kathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Spees",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Marta",
"given": "Mailyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Amir",
"given": "Daniah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Collazo",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Dolor",
"given": "Rowena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vergara",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Jordan",
"given": "Jackie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Burruss",
"given": "Valencia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Hurst",
"given": "Terri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rebolledo Esteinou",
"given": "Paulina A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ofotokun",
"given": "Igho",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Zhang",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Traenkner",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Atha",
"given": "Mary M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "James",
"given": "Vickie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rogers",
"given": "Marcella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Oragwu",
"given": "Chukwuemeka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Oguego",
"given": "Ngozi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pillai",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gabriel",
"given": "Ahab",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ghaly",
"given": "Emad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Michal",
"given": "Marian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Hassanien",
"given": "Gammal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ismail",
"given": "Samah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Samir",
"given": "Yehia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Meltzer",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Heidish",
"given": "Ryan S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Loganathan",
"given": "Aditya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Brehaut",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Roche",
"given": "Angelina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Koppinger",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Baez",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pagan",
"given": "Ivone",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Abdelsayed",
"given": "Dallal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Aziz",
"given": "Mina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Robinson",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lozinski",
"given": "Grace",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nguyen",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Griffin",
"given": "Alvin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Morris",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Love",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mattox",
"given": "Bonnie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Martin",
"given": "Raykel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pardue",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rowland",
"given": "Teddy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Reyes",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bacallao",
"given": "Navila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cienki",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Yuan",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Li",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Szeto",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Stelmash",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mekhael",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Morales Castillo",
"given": "Ledular",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gutierrez",
"given": "Anya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Prieto",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Amon",
"given": "Arch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Barbera",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bugajski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wills",
"given": "Walter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Jacklin",
"given": "Kellcee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lamb",
"given": "Deryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Harper",
"given": "Amron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Stout",
"given": "Elmer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Griffin",
"given": "Merischia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Clark",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Barsanti-Sekhar",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Carbrera-Mendez",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Evans",
"given": "Mary Rose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Maria",
"given": "Josette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Raymond",
"given": "Oksana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Summers",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Turner",
"given": "Tammy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lenert",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Panaccione",
"given": "Ebony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Miller",
"given": "Conrad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wiley",
"given": "Hawa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Chan",
"given": "Austin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Khizer",
"given": "Saadia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Adeyemi",
"given": "Oluwadamilola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ning Chi",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Chen",
"given": "July",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Morton-Jost",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Castex",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Quirch",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Belani",
"given": "Hrishikesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Machicado",
"given": "Rosario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bjornsson",
"given": "Bjorn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Olivo",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Maldonado",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vecchiarelli",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gaytan-Alvarez",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cherukuri",
"given": "Vijaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Alicic",
"given": "Radica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lambert",
"given": "Allison A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Urbat",
"given": "Carissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Baxter",
"given": "Joni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cooper",
"given": "Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Linn",
"given": "Dawn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Fisher",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Patel",
"given": "Vijay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Patel",
"given": "Yuti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Talati",
"given": "Roshan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Patel",
"given": "Priti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ellison",
"given": "Leonard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Roman",
"given": "Angee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Harrison",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Moy",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Naquiallah",
"given": "Dina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Shah",
"given": "Binod",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Jagannathan",
"given": "Prasanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "O’Donnell",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Jazayeri",
"given": "Yasmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gupta",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Chandrasekar",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Curtis",
"given": "Clifford",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "White",
"given": "Briana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Dockery",
"given": "Martha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Fortt",
"given": "Tabitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Fortt",
"given": "Anisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wilson",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Marcelin",
"given": "Jackie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Farlow",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Grady",
"given": "Casey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Richwine",
"given": "Randall",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pazier",
"given": "Penny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Michelson",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Watts",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Kariyawasam",
"given": "Diluma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Rodriguez",
"given": "Leann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Luis Garcia",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Manresa",
"given": "Ismarys",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Achong",
"given": "Angel A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Garcia",
"given": "Mari C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mahadevan",
"given": "Arvind",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "VandeWeerd",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lowenkron",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Sappington",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Roberts",
"given": "Mitchell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wang",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Adams",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ding",
"given": "Xinyi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Co",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "D'Andrea",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Lim",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Young",
"given": "Madeline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wilson",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Eastin",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cheathem",
"given": "Allyson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nadeem",
"given": "Ahad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Walters",
"given": "Crystal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Powers-Fletcher",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Brown",
"given": "Douglas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Miller",
"given": "Delia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Mukunzi",
"given": "Sylvere",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Isache",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bowman",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Martin",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Duran",
"given": "Brent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Schwasinger-Schmidt",
"given": "Tiffany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ast",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Cornejo",
"given": "Ashlie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Archer",
"given": "Allie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Almanzar",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Motel",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pullen",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Bhat",
"given": "Neeta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Parra",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Urbina",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Arriaza",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Sekikawa",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Klawson",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Weiland",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ostrosky-Zeichner",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Umana",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Nielsen",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Grimes",
"given": "Carolyn Z.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Patterson",
"given": "Thomas F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Soileau",
"given": "Bridgette T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Jackson",
"given": "Patrick E.H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Haughey",
"given": "Heather M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Vaidya-Tank",
"given": "Bhavna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Gould",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Goyal",
"given": "Parul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Pangburn",
"given": "Haley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Michalowski",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Williamson",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Wortham",
"given": "Brittany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Abbott",
"given": "Rica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Umana",
"given": "Unwana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Alleyne",
"given": "Candace",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Witting",
"given": "Britta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Armas",
"given": "Eddie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Perez Landaburo",
"given": "Ramon O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "De La Cruz",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Ballmajo",
"given": "Martha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Accelerating COVID-19 Therapeutic Interventions and Vaccines–6 Study Group and Investigators"
}
],
"family": "Alvarez",
"given": "Jorge",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
7,
14
]
],
"date-time": "2025-07-14T15:00:44Z",
"timestamp": 1752505244000
},
"deposited": {
"date-parts": [
[
2025,
7,
14
]
],
"date-time": "2025-07-14T15:00:45Z",
"timestamp": 1752505245000
},
"indexed": {
"date-parts": [
[
2025,
7,
14
]
],
"date-time": "2025-07-14T15:40:07Z",
"timestamp": 1752507607668,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
7,
14
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2836528/jamainternal_bramante_2025_oi_250040_1752124349.53557.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2025,
7,
14
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
14
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1371/journal.pone.0191805",
"article-title": "Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy.",
"author": "Del Campo",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "PLoS One",
"key": "ioi250040r1",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1002/jmv.26728",
"article-title": "mTOR inhibition in COVID-19: A commentary and review of efficacy in RNA viruses.",
"author": "Karam",
"doi-asserted-by": "publisher",
"first-page": "1843",
"issue": "4",
"journal-title": "J Med Virol",
"key": "ioi250040r2",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.18632/oncotarget.20248",
"article-title": "Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase.",
"author": "Tsai",
"doi-asserted-by": "publisher",
"first-page": "91928",
"issue": "54",
"journal-title": "Oncotarget",
"key": "ioi250040r3",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1371/journal.ppat.1006257",
"article-title": "DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: a potential antiviral target.",
"author": "Soto-Acosta",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "PLoS Pathog",
"key": "ioi250040r4",
"volume": "13",
"year": "2017"
},
{
"DOI": "10.1172/JCI170236",
"article-title": "Preparing for the next viral threat with broad-spectrum antivirals.",
"author": "Karim",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "J Clin Invest",
"key": "ioi250040r5",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.3390/ph16091279",
"article-title": "Metformin hydrochloride significantly inhibits rotavirus infection in Caco2 cell line, intestinal organoids, and mice.",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "1279",
"issue": "9",
"journal-title": "Pharmaceuticals (Basel)",
"key": "ioi250040r6",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.3389/fendo.2021.587801",
"article-title": "Metformin and Covid-19: focused review of mechanisms and current literature suggesting benefit.",
"author": "Ibrahim",
"doi-asserted-by": "publisher",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "ioi250040r7",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/j.1348-0421.2011.00382.x",
"article-title": "Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways.",
"author": "Nakashima",
"doi-asserted-by": "publisher",
"first-page": "774",
"issue": "11",
"journal-title": "Microbiol Immunol",
"key": "ioi250040r8",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.1016/j.bbrc.2013.01.103",
"article-title": "Unfolded protein response pathways regulate hepatitis C virus replication via modulation of autophagy.",
"author": "Shinohara",
"doi-asserted-by": "publisher",
"first-page": "326",
"issue": "2",
"journal-title": "Biochem Biophys Res Commun",
"key": "ioi250040r9",
"volume": "432",
"year": "2013"
},
{
"DOI": "10.1002/jmv.26498",
"article-title": "Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: a meta-analysis.",
"author": "Kow",
"doi-asserted-by": "publisher",
"first-page": "695",
"issue": "2",
"journal-title": "J Med Virol",
"key": "ioi250040r10",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s43441-024-00633-6",
"article-title": "The effect of antihyperglycemic medications on COVID-19: a meta-analysis and systematic review from observational studies.",
"author": "Song",
"doi-asserted-by": "publisher",
"first-page": "773",
"issue": "4",
"journal-title": "Ther Innov Regul Sci",
"key": "ioi250040r11",
"volume": "58",
"year": "2024"
},
{
"DOI": "10.3389/fendo.2020.600439",
"article-title": "Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes.",
"author": "Crouse",
"doi-asserted-by": "publisher",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "ioi250040r12",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19.",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "599",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "ioi250040r13",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2022.113223",
"article-title": "Treatment with metformin glycinate reduces SARS-CoV-2 viral load: an in vitro model and randomized, double-blind, phase IIb clinical trial.",
"author": "Ventura-López",
"doi-asserted-by": "publisher",
"journal-title": "Biomed Pharmacother",
"key": "ioi250040r14",
"volume": "152",
"year": "2022"
},
{
"DOI": "10.1016/j.diabres.2022.110157",
"article-title": "Metformin is associated with reduced COVID-19 severity in patients with prediabetes.",
"author": "Chan",
"doi-asserted-by": "publisher",
"journal-title": "Diabetes Res Clin Pract",
"key": "ioi250040r15",
"volume": "194",
"year": "2022"
},
{
"DOI": "10.1016/j.gloepi.2024.100142",
"article-title": "Estimating the effect of realistic improvements of metformin adherence on COVID-19 mortality using targeted machine learning.",
"author": "Qiu",
"doi-asserted-by": "publisher",
"journal-title": "Glob Epidemiol",
"key": "ioi250040r16",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1007/s11606-022-07701-3",
"article-title": "Medications associated with lower mortality in a SARS-CoV-2 positive cohort of 26,508 veterans.",
"author": "Hunt",
"doi-asserted-by": "publisher",
"first-page": "4144",
"issue": "16",
"journal-title": "J Gen Intern Med",
"key": "ioi250040r17",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1007/s11239-022-02631-7",
"article-title": "Metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes.",
"author": "Usman",
"doi-asserted-by": "publisher",
"first-page": "363",
"issue": "2",
"journal-title": "J Thromb Thrombolysis",
"key": "ioi250040r18",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.1017/cts.2023.644",
"article-title": "ACTIV-6: operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19.",
"author": "Accelerating Covid-19 Therapeutic Interventions and Vaccines–6 Study Group",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Clin Transl Sci",
"key": "ioi250040r19",
"volume": "7",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.18590",
"article-title": "Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial.",
"author": "Naggie",
"doi-asserted-by": "publisher",
"first-page": "1595",
"issue": "16",
"journal-title": "JAMA",
"key": "ioi250040r20",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1186/1745-6215-11-32",
"article-title": "CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.",
"author": "Schulz",
"doi-asserted-by": "publisher",
"first-page": "32",
"journal-title": "Trials",
"key": "ioi250040r21",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners.",
"author": "Harris",
"doi-asserted-by": "publisher",
"journal-title": "J Biomed Inform",
"key": "ioi250040r22",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support.",
"author": "Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "ioi250040r23",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1002/sim.10133",
"article-title": "Bayesian transition models for ordinal longitudinal outcomes.",
"author": "Rohde",
"doi-asserted-by": "publisher",
"first-page": "3539",
"issue": "18",
"journal-title": "Stat Med",
"key": "ioi250040r24",
"volume": "43",
"year": "2024"
},
{
"DOI": "10.1001/jama.2022.21506",
"article-title": "Adjustment for baseline characteristics in randomized clinical trials.",
"author": "Holmberg",
"doi-asserted-by": "publisher",
"first-page": "2155",
"issue": "21",
"journal-title": "JAMA",
"key": "ioi250040r26",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciae284",
"article-title": "Is there room for metformin at COVID-19’s dinner table? updated analysis of clinical trials.",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "487",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "ioi250040r27",
"volume": "80",
"year": "2025"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "ioi250040r28",
"volume": "386",
"year": "2022"
},
{
"article-title": "Expression of concern—effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial.",
"author": "Lancet",
"journal-title": "Lancet Reg Health Am",
"key": "ioi250040r29",
"volume": "31",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciae257",
"article-title": "Correction to: repurposing revisited: exploring the role of metformin for treatment of coronavirus disease 2019.",
"doi-asserted-by": "publisher",
"first-page": "285",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "ioi250040r30",
"volume": "79",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients.",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "ioi250040r31",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7342a1",
"article-title": "Genomic surveillance for SARS-CoV-2 variants: circulation of omicron XBB and JN.1 lineages—United States, May 2023-September 2024.",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "938",
"issue": "42",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ioi250040r32",
"volume": "73",
"year": "2024"
},
{
"DOI": "10.1038/s41467-024-52668-w",
"article-title": "Immune escape and attenuated severity associated with the SARS-CoV-2 BA.2.86/JN.1 lineage.",
"author": "Lewnard",
"doi-asserted-by": "publisher",
"first-page": "8550",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ioi250040r33",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1093/infdis/jiae364",
"article-title": "Reduced likelihood of hospitalization with the JN.1 or HV.1 SARS-CoV-2 variants compared to the EG.5 variant.",
"author": "Levy",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "J Infect Dis",
"key": "ioi250040r34",
"volume": "230",
"year": "2024"
},
{
"DOI": "10.1056/NEJMra1510064",
"article-title": "The primary outcome fails—what next?",
"author": "Pocock",
"doi-asserted-by": "publisher",
"first-page": "861",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "ioi250040r35",
"volume": "375",
"year": "2016"
},
{
"DOI": "10.1016/j.chom.2022.11.012",
"article-title": "Enhanced neutralization resistance of SARS-CoV-2 omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2.",
"author": "Qu",
"doi-asserted-by": "publisher",
"first-page": "9",
"issue": "1",
"journal-title": "Cell Host Microbe",
"key": "ioi250040r36",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1080/22221751.2024.2402880",
"article-title": "Recurrent SARS-CoV-2 spike mutations confer growth advantages to select JN.1 sublineages.",
"author": "Wang",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "ioi250040r37",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2301425",
"article-title": "Oral simnotrelvir for adult patients with mild-to-moderate Covid-19.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "230",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "ioi250040r38",
"volume": "390",
"year": "2024"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing.",
"author": "Gordon",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "7816",
"journal-title": "Nature",
"key": "ioi250040r39",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2022.199010",
"article-title": "Metformin suppresses SARS-CoV-2 in cell culture.",
"author": "Parthasarathy",
"doi-asserted-by": "publisher",
"journal-title": "Virus Res",
"key": "ioi250040r40",
"volume": "323",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciae159",
"article-title": "Favorable antiviral effect of metformin on severe acute respiratory syndrome coronavirus 2 viral load in a randomized, placebo-controlled clinical trial of coronavirus disease 2019.",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "354",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "ioi250040r41",
"volume": "79",
"year": "2024"
},
{
"DOI": "10.1172/jci.insight.148003",
"article-title": "Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses.",
"author": "Schaller",
"doi-asserted-by": "publisher",
"issue": "18",
"journal-title": "JCI Insight",
"key": "ioi250040r42",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.immuni.2021.05.004",
"article-title": "Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation.",
"author": "Xian",
"doi-asserted-by": "publisher",
"first-page": "1463",
"issue": "7",
"journal-title": "Immunity",
"key": "ioi250040r43",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial.",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "1119",
"issue": "10",
"journal-title": "Lancet Infect Dis",
"key": "ioi250040r44",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofae631.016",
"article-title": "Metformin reduces the risk of Long COVID or death over 6 months in an emulated target trial of primarily omicron-infected adults without diabetes or prediabetes: a new-user, active-comparator analysis using the National COVID Cohort Collaborative (N3C).",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "S10",
"journal-title": "Open Forum Infectious Diseases",
"key": "ioi250040r45",
"volume": "12",
"year": "2025"
},
{
"key": "ioi250040r25",
"unstructured": "US Food and Drug Administration. Adjusting for covariates in randomized clinical trials for drugs and biological products. 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/adjusting-covariates-randomized-clinical-trials-drugs-and-biological-products"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2836528"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"The ACTIV-6 Randomized Clinical Trial"
],
"title": "Metformin and Time to Sustained Recovery in Adults With COVID-19",
"type": "journal-article"
}