Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2–Positive Patients: A Randomized Controlled Clinical Trial
et al., Journal of Dental Research, doi:10.1177/00220345231156415, DRKS00027812, Mar 2023
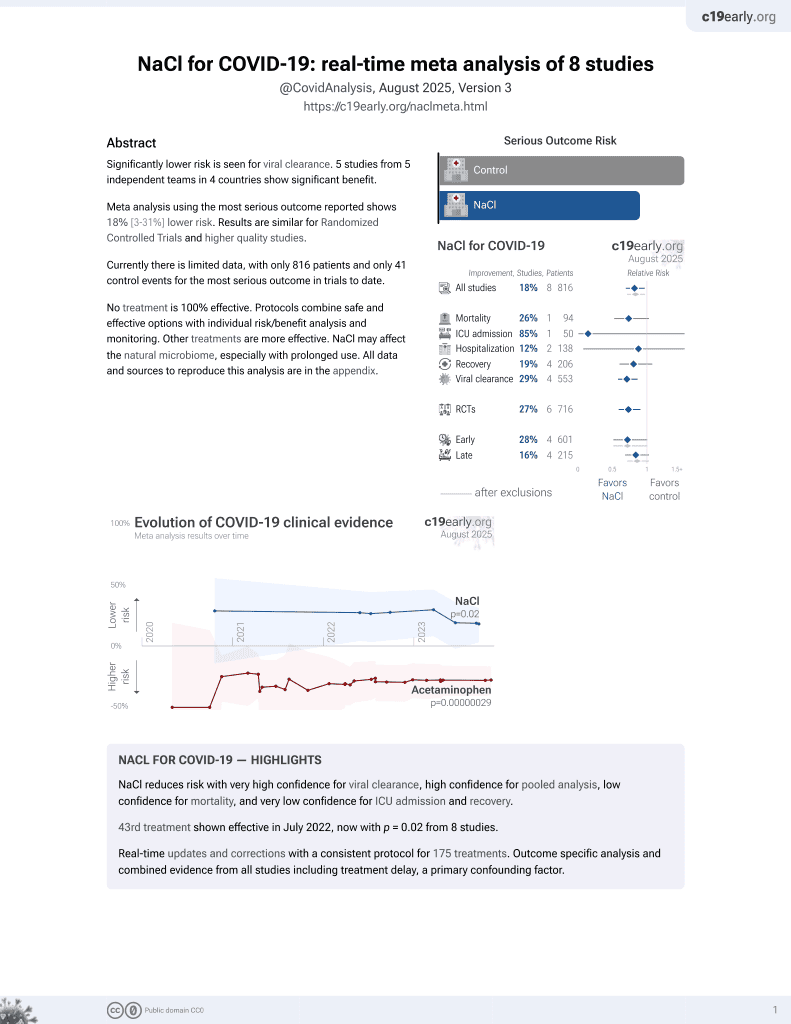
NaCl for COVID-19
44th treatment shown to reduce risk in
July 2022, now with p = 0.0028 from 9 studies.
Lower risk for progression and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 61 COVID+ patients showing a significant reduction in viral load and infectivity with a mouthwash containing 0.05% cetylpyridinium chloride (CPC) and 0.05% chlorhexidine digluconate (CHX). Mouthwash containing 0.9% NaCl showed a trend towards lower infectivity. The study only analyzes short-term changes in viral load 30 minutes after treatment.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Study covers chlorhexidine, cetylpyridinium chloride, and NaCl.
|
viral load, 71.7% lower, relative load 0.28, p = 0.10, treatment median 1.7 IQR 2.3 n=9, control median 6.0 IQR 49.0 n=9, relative infectious viral load, 30 min vs. baseline.
|
|
viral load, 70.6% lower, relative load 0.29, p = 0.53, treatment 30, control 30, relative PCR viral load, 30 min vs. baseline.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bonn et al., 21 Mar 2023, prospective, Germany, peer-reviewed, 14 authors, study period 4 January, 2022 - 22 February, 2022, trial DRKS00027812.
Contact: fabian.cieplik@ukr.de.
DOI record:
{
"DOI": "10.1177/00220345231156415",
"ISSN": [
"0022-0345",
"1544-0591"
],
"URL": "http://dx.doi.org/10.1177/00220345231156415",
"abstract": "<jats:p> Soon after the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, preprocedural mouthwashes were recommended for temporarily reducing intraoral viral load and infectivity of individuals potentially infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in order to protect medical personnel. Particularly, the antiseptic cetylpyridinium chloride (CPC) has shown virucidal effects against SARS-CoV-2 in vitro. Therefore, the aim of this randomized controlled clinical trial was to investigate the efficacy of a commercially available mouthwash containing CPC and chlorhexidine digluconate (CHX) at 0.05% each in SARS-CoV-2–positive patients as compared to a placebo mouthwash. Sixty-one patients who tested positive for SARS-CoV-2 with onset of symptoms within the last 72 h were included in this study. Oropharyngeal specimens were taken at baseline, whereupon patients had to gargle mouth and throat with 20 mL test or placebo (0.9% NaCl) mouthwash for 60 s. After 30 min, further oropharyngeal specimens were collected. Viral load was analyzed by quantitative reverse transcriptase polymerase chain reaction, and infectivity of oropharyngeal specimens was analyzed by virus rescue in cell culture and quantified via determination of tissue culture infectious doses 50% (TCID<jats:sub>50</jats:sub>). Data were analyzed nonparametrically (α = 0.05). Viral load slightly but significantly decreased upon gargling in the test group ( P = 0.0435) but not in the placebo group. Viral infectivity as measured by TCID<jats:sub>50</jats:sub> also significantly decreased in the test group ( P = 0.0313), whereas there was no significant effect but a trend in the placebo group. Furthermore, it was found that the specimens from patients with a vaccine booster exhibited significantly lower infectivity at baseline as compared to those without vaccine booster ( P = 0.0231). This study indicates that a preprocedural mouthwash containing CPC and CHX could slightly but significantly reduce the viral load and infectivity in SARS-CoV-2–positive patients. Further studies are needed to corroborate these results and investigate whether the observed reductions in viral load and infectivity could translate into clinically useful effects in reducing COVID-19 transmission (German Clinical Trials Register DRKS00027812). </jats:p>",
"alternative-id": [
"10.1177/00220345231156415"
],
"author": [
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
},
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
}
],
"family": "Bonn",
"given": "E.L.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Rohrhofer",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Praxiszentrum Alte Mälzerei, Regensburg, Germany"
}
],
"family": "Audebert",
"given": "F.X.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Praxiszentrum Alte Mälzerei, Regensburg, Germany"
}
],
"family": "Lang",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Auer",
"given": "D.L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Scholz",
"given": "K.J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
}
],
"family": "Schuster",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Wenzel",
"given": "J.J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Hiller",
"given": "K.-A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Buchalla",
"given": "W.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3623-3183",
"affiliation": [
{
"name": "Department of Oral and Maxillofacial Surgery, University Hospital Regensburg, Regensburg, Germany"
}
],
"authenticated-orcid": false,
"family": "Gottsauner",
"given": "J.M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otorhinolaryngology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Vielsmeier",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
},
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Schmidt",
"given": "B.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1750-7380",
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"authenticated-orcid": false,
"family": "Cieplik",
"given": "F.",
"sequence": "additional"
}
],
"container-title": "Journal of Dental Research",
"container-title-short": "J Dent Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T07:10:17Z",
"timestamp": 1679382617000
},
"deposited": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T13:57:46Z",
"timestamp": 1740837466000
},
"funder": [
{
"name": "Dentaid S.L., Barcelona, Spain"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
4
]
],
"date-time": "2025-04-04T08:45:15Z",
"timestamp": 1743756315361,
"version": "3.38.0"
},
"is-referenced-by-count": 15,
"issue": "6",
"issued": {
"date-parts": [
[
2023,
3,
21
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2023,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T00:00:00Z",
"timestamp": 1679356800000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00220345231156415",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/00220345231156415",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00220345231156415",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "608-615",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
21
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1177/00220345221102310",
"doi-asserted-by": "publisher",
"key": "bibr1-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2021.03.021",
"doi-asserted-by": "publisher",
"key": "bibr2-00220345231156415"
},
{
"DOI": "10.3390/antibiotics11050688",
"doi-asserted-by": "publisher",
"key": "bibr3-00220345231156415"
},
{
"DOI": "10.1080/22221751.2022.2098059",
"doi-asserted-by": "publisher",
"key": "bibr4-00220345231156415"
},
{
"DOI": "10.1111/jopr.13220",
"doi-asserted-by": "publisher",
"key": "bibr5-00220345231156415"
},
{
"DOI": "10.1093/infdis/jiab415",
"doi-asserted-by": "publisher",
"key": "bibr6-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2021.05.021",
"doi-asserted-by": "publisher",
"key": "bibr7-00220345231156415"
},
{
"DOI": "10.1177/00220345221110444",
"doi-asserted-by": "publisher",
"key": "bibr8-00220345231156415"
},
{
"DOI": "10.3389/fmicb.2019.00587",
"doi-asserted-by": "publisher",
"key": "bibr9-00220345231156415"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "bibr10-00220345231156415"
},
{
"author": "Domênico MBD",
"journal-title": "Epidemiol Health",
"key": "bibr11-00220345231156415",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"doi-asserted-by": "publisher",
"key": "bibr12-00220345231156415"
},
{
"DOI": "10.1093/cid/ciaa1616",
"doi-asserted-by": "publisher",
"key": "bibr13-00220345231156415"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "bibr14-00220345231156415"
},
{
"DOI": "10.1016/S2213-2600(22)00226-0",
"doi-asserted-by": "publisher",
"key": "bibr15-00220345231156415"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"doi-asserted-by": "publisher",
"key": "bibr16-00220345231156415"
},
{
"DOI": "10.1002/jmv.26954",
"doi-asserted-by": "publisher",
"key": "bibr17-00220345231156415"
},
{
"DOI": "10.1111/prd.12361",
"doi-asserted-by": "publisher",
"key": "bibr18-00220345231156415"
},
{
"DOI": "10.20411/pai.v6i1.441",
"doi-asserted-by": "publisher",
"key": "bibr19-00220345231156415"
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "publisher",
"key": "bibr20-00220345231156415"
},
{
"DOI": "10.1177/0022034520943574",
"doi-asserted-by": "publisher",
"key": "bibr21-00220345231156415"
},
{
"DOI": "10.1128/AAC.00576-20",
"doi-asserted-by": "publisher",
"key": "bibr22-00220345231156415"
},
{
"DOI": "10.3389/fmicb.2022.934525",
"doi-asserted-by": "publisher",
"key": "bibr23-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2019.06.024",
"doi-asserted-by": "publisher",
"key": "bibr24-00220345231156415"
},
{
"DOI": "10.1093/infdis/jiaa471",
"doi-asserted-by": "publisher",
"key": "bibr25-00220345231156415"
},
{
"DOI": "10.1016/j.virusres.2022.198791",
"doi-asserted-by": "publisher",
"key": "bibr26-00220345231156415"
},
{
"DOI": "10.1177/0022034520914246",
"doi-asserted-by": "publisher",
"key": "bibr27-00220345231156415"
},
{
"DOI": "10.1007/s00784-021-04363-z",
"doi-asserted-by": "publisher",
"key": "bibr28-00220345231156415"
},
{
"DOI": "10.1016/S1473-3099(21)00472-2",
"doi-asserted-by": "publisher",
"key": "bibr29-00220345231156415"
},
{
"DOI": "10.3390/microorganisms10030561",
"doi-asserted-by": "publisher",
"key": "bibr30-00220345231156415"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "bibr31-00220345231156415"
},
{
"DOI": "10.1016/S2468-2667(20)30164-X",
"doi-asserted-by": "publisher",
"key": "bibr32-00220345231156415"
},
{
"DOI": "10.1038/s41368-020-0075-9",
"doi-asserted-by": "publisher",
"key": "bibr33-00220345231156415"
},
{
"DOI": "10.1177/2380084421993099",
"doi-asserted-by": "publisher",
"key": "bibr34-00220345231156415"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "publisher",
"key": "bibr35-00220345231156415"
},
{
"DOI": "10.1111/imr.13089",
"doi-asserted-by": "publisher",
"key": "bibr36-00220345231156415"
},
{
"DOI": "10.1056/NEJMc2004973",
"doi-asserted-by": "publisher",
"key": "bibr37-00220345231156415"
},
{
"DOI": "10.1038/s41598-019-44822-y",
"doi-asserted-by": "publisher",
"key": "bibr38-00220345231156415"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "bibr39-00220345231156415"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "bibr40-00220345231156415"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/00220345231156415"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2–Positive Patients: A Randomized Controlled Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1177/sage-journals-update-policy",
"volume": "102"
}