Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2–Positive Patients: A Randomized Controlled Clinical Trial
et al., Journal of Dental Research, doi:10.1177/00220345231156415, DRKS00027812, Mar 2023
53rd treatment shown to reduce risk in
November 2023, now with p < 0.00000000001 from 5 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
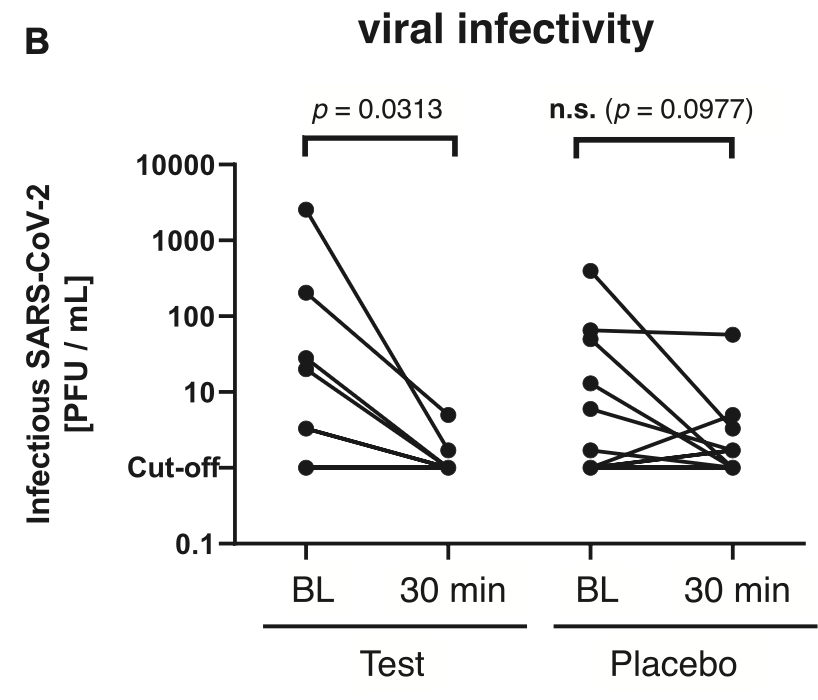
RCT 61 COVID+ patients showing improved reduction in viral infectivity with a mouthwash containing 0.05% cetylpyridinium chloride (CPC) and 0.05% chlorhexidine digluconate (CHX), compared to 0.9% NaCl. The study only analyzes short-term changes in viral load 30 minutes after treatment.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Study covers chlorhexidine, cetylpyridinium chloride, and NaCl.
|
viral load, 85.3% lower, relative load 0.15, p = 0.20, treatment 6, control 9, relative reduction in infectious viral load, 30 min vs. baseline, CPC+CHX.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bonn et al., 21 Mar 2023, Double Blind Randomized Controlled Trial, Germany, peer-reviewed, 14 authors, study period 4 January, 2022 - 22 February, 2022, this trial uses multiple treatments in the treatment arm (combined with CPC) - results of individual treatments may vary, trial DRKS00027812.
Contact: fabian.cieplik@ukr.de.
Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2–Positive Patients: A Randomized Controlled Clinical Trial
Journal of Dental Research, doi:10.1177/00220345231156415
Soon after the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, preprocedural mouthwashes were recommended for temporarily reducing intraoral viral load and infectivity of individuals potentially infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in order to protect medical personnel. Particularly, the antiseptic cetylpyridinium chloride (CPC) has shown virucidal effects against SARS-CoV-2 in vitro. Therefore, the aim of this randomized controlled clinical trial was to investigate the efficacy of a commercially available mouthwash containing CPC and chlorhexidine digluconate (CHX) at 0.05% each in SARS-CoV-2-positive patients as compared to a placebo mouthwash. Sixty-one patients who tested positive for SARS-CoV-2 with onset of symptoms within the last 72 h were included in this study. Oropharyngeal specimens were taken at baseline, whereupon patients had to gargle mouth and throat with 20 mL test or placebo (0.9% NaCl) mouthwash for 60 s. After 30 min, further oropharyngeal specimens were collected. Viral load was analyzed by quantitative reverse transcriptase polymerase chain reaction, and infectivity of oropharyngeal specimens was analyzed by virus rescue in cell culture and quantified via determination of tissue culture infectious doses 50% (TCID 50 ). Data were analyzed nonparametrically (α = 0.05). Viral load slightly but significantly decreased upon gargling in the test group (P = 0.0435) but not in the placebo group. Viral infectivity as measured by TCID 50 also significantly decreased in the test group (P = 0.0313), whereas there was no significant effect but a trend in the placebo group. Furthermore, it was found that the specimens from patients with a vaccine booster exhibited significantly lower infectivity at baseline as compared to those without vaccine booster (P = 0.0231). This study indicates that a preprocedural mouthwash containing CPC and CHX could slightly but significantly reduce the viral load and infectivity in SARS-CoV-2-positive patients. Further studies are needed to corroborate these results and investigate whether the observed reductions in viral load and infectivity could translate into clinically useful effects in reducing COVID-19 transmission (German Clinical Trials Register DRKS00027812).
Author Contributions E.L. Bonn, contributed to data acquisition, analysis and interpretation, drafted and critically revised the manuscript; A. Rohrhofer, contributed to data analysis, critically revised the manuscript; F.-X. Audebert, contributed to conception and design, data acquisition and interpretation, critically revised the manuscript; H. Lang, contributed to conception and design, data acquisition, critically revised the manuscript; D.L. Auer, contributed to acquisition, analysis and interpretation, critically revised the manuscript; K.J. Scholz, P. Schuster, B. Schmidt, contributed to conception and design, data analysis and interpretation, critically revised the manuscript; J.J. Wenzel, K.-A. Hiller, contributed to data analysis and interpretation, critically revised the manuscript; W. Buchalla, J.-M. Gottsauner, V. Vielsmeier, contributed to conception and design, data interpretation, critically revised the manuscript; F. Cieplik, contributed to conception and design, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was..
References
Alemany, Perez-Zsolt, Raïch-Regué, Muñoz-Basagoiti, Ouchi et al., Cetylpyridinium chloride mouthwash to reduce shedding of infectious SARS-CoV-2: a double-blind randomized clinical trial, J Dent Res
Araujo, Estrich, Mikkelsen, Morrissey, Harrison et al., COVID-2019 among dentists in the United States: a 6-month longitudinal report of accumulative prevalence and incidence, J Am Dent Assoc
Auer, Mao, Anderson, Muehler, Wittmer et al., Phenotypic adaptation to antiseptics and effects on biofilm formation capacity and antibiotic resistance in clinical isolates of early colonizers in dental plaque, Antibiotics
Barrueco, Mateos-Moreno, Martínez-Beneyto, García-Vázquez, González et al., Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial, Emerg Microbes Infec
Bidra, Pelletier, Westover, Frank, Brown et al., Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses, J Prosthodont
Buder, Bauswein, Magnus, Audebert, Lang et al., SARS-CoV-2 infectivity correlates with high viral loads and detection of viral antigen and is terminated by seroconversion, J Infect Dis
Chaudhary, Melkonyan, Meethil, Saraswat, Hall et al., Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load, J Am Dent Assoc
Cieplik, Jakubovics, Buchalla, Maisch, Hellwig, Resistance toward chlorhexidine in oral bacteria-is there cause for concern?, Front Microbiol
Cieplik, Jakubovics, Preprocedural mouthwashes for reduction of SARS-CoV-2 viral load and infectivity, J Dent Res
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill
Domênico, Cesca, Ponciano, Santos, Lenz et al., Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial, Epidemiol Health
Ferrer, Barrueco, Martinez-Beneyto, Moreno, Ausina-Márquez et al., Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Sci Rep
Gniazdowski, Morris, Wohl, Mehoke, Ramakrishnan et al., Repeated coronavirus disease 2019 molecular testing: correlation of severe acute respi-ratory syndrome coronavirus 2 culture with molecular assays and cycle thresholds, Clin Infect Dis
Gottsauner, Michaelides, Schmidt, Scholz, Buchalla et al., A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin Oral Investig
Hakki, Zhou, Jonnerby, Singanayagam, Barnett et al., Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study, Lancet Respir Med
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin Oral Invest
Huang, Huang, Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients, J Med Virol
Jakubovics, Goodman, Mashburn-Warren, Stafford, Cieplik, The dental plaque biofilm matrix, Periodontol
Ketas, Chaturbhuj, Portillo, Francomano, Golden et al., Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva, Pathogens Immun
Koch-Heier, Hoffmann, Schindler, Lussi, Planz, Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX ® and BacterX ® Pro, Microorganisms
Koletsi, Belibasakis, Eliades, Interventions to reduce aerosolized microbes in dental practice: a systematic review with network metaanalysis of randomized controlled trials, J Dent Res
Mao, Auer, Buchalla, Hiller, Maisch et al., Cetylpyridinium chloride: mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance, Antimicrob Agents Chemother
Mao, Hiergeist, Auer, Scholz, Muehler et al., Ecological effects of daily antiseptic treatment on microbial composition of saliva-grown microcosm biofilms and selection of resistant phenotypes, Front Microbiol
Marui, Souto, Rovai, Romito, Chambrone et al., Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review, J Am Dent Assoc
Meister, Brüggemann, Todt, Conzelmann, Müller et al., Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis
Meister, Gottsauner, Schmidt, Heinen, Todt et al., Mouthrinses against SARS-CoV-2-high antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial, Virus Res
Meng, Hua, Bian, Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine, J Dent Res
Mksoud, Ittermann, Holtfreter, Söhnel, Söhnel et al., Prevalence of SARS-CoV-2 IgG antibodies among dental teams in Germany, Clin Oral Invest
Mostaghimi, Valdez, Larson, Kalinich, Iwasaki, Prevention of host-to-host transmission by SARS-CoV-2 vaccines, Lancet Infect Dis
Muehler, Mao, Czemmel, Geißert, Engesser et al., Transcriptomic stress response in Streptococcus mutans following treatment with a sublethal concentration of chlorhexidine digluconate, Microorganisms
Muñoz-Basagoiti, Perez-Zsolt, León, Blanc, Raïch-Regué et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, J Dent Res
Nguyen, Drew, Graham, Joshi, Guo et al., Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study, Lancet Public Health
Peng, Xu, Li, Cheng, Zhou et al., Transmission routes of 2019-nCoV and controls in dental practice, Int J Oral Sci
Sarapultseva, Hu, Sarapultsev, SARS-CoV-2 seropositivity among dental staff and the role of aspirating systems, JDR Clin Transl Res
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Sette, Crotty, Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines, Immunol Rev
Van Doremalen, Bushmaker, Morris, Holbrook, Gamble et al., Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, New Engl J Med
Verspecht, Herrero, Khodaparast, Khodaparast, Boon et al., Development of antiseptic adaptation and cross-adaptation in selected oral pathogens in vitro, Sci Rep
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, New Engl J Med
DOI record:
{
"DOI": "10.1177/00220345231156415",
"ISSN": [
"0022-0345",
"1544-0591"
],
"URL": "http://dx.doi.org/10.1177/00220345231156415",
"abstract": "<jats:p> Soon after the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, preprocedural mouthwashes were recommended for temporarily reducing intraoral viral load and infectivity of individuals potentially infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in order to protect medical personnel. Particularly, the antiseptic cetylpyridinium chloride (CPC) has shown virucidal effects against SARS-CoV-2 in vitro. Therefore, the aim of this randomized controlled clinical trial was to investigate the efficacy of a commercially available mouthwash containing CPC and chlorhexidine digluconate (CHX) at 0.05% each in SARS-CoV-2–positive patients as compared to a placebo mouthwash. Sixty-one patients who tested positive for SARS-CoV-2 with onset of symptoms within the last 72 h were included in this study. Oropharyngeal specimens were taken at baseline, whereupon patients had to gargle mouth and throat with 20 mL test or placebo (0.9% NaCl) mouthwash for 60 s. After 30 min, further oropharyngeal specimens were collected. Viral load was analyzed by quantitative reverse transcriptase polymerase chain reaction, and infectivity of oropharyngeal specimens was analyzed by virus rescue in cell culture and quantified via determination of tissue culture infectious doses 50% (TCID<jats:sub>50</jats:sub>). Data were analyzed nonparametrically (α = 0.05). Viral load slightly but significantly decreased upon gargling in the test group ( P = 0.0435) but not in the placebo group. Viral infectivity as measured by TCID<jats:sub>50</jats:sub> also significantly decreased in the test group ( P = 0.0313), whereas there was no significant effect but a trend in the placebo group. Furthermore, it was found that the specimens from patients with a vaccine booster exhibited significantly lower infectivity at baseline as compared to those without vaccine booster ( P = 0.0231). This study indicates that a preprocedural mouthwash containing CPC and CHX could slightly but significantly reduce the viral load and infectivity in SARS-CoV-2–positive patients. Further studies are needed to corroborate these results and investigate whether the observed reductions in viral load and infectivity could translate into clinically useful effects in reducing COVID-19 transmission (German Clinical Trials Register DRKS00027812). </jats:p>",
"alternative-id": [
"10.1177/00220345231156415"
],
"author": [
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
},
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
}
],
"family": "Bonn",
"given": "E.L.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Rohrhofer",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Praxiszentrum Alte Mälzerei, Regensburg, Germany"
}
],
"family": "Audebert",
"given": "F.X.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Praxiszentrum Alte Mälzerei, Regensburg, Germany"
}
],
"family": "Lang",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Auer",
"given": "D.L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Scholz",
"given": "K.J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
}
],
"family": "Schuster",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Wenzel",
"given": "J.J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Hiller",
"given": "K.-A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Buchalla",
"given": "W.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3623-3183",
"affiliation": [
{
"name": "Department of Oral and Maxillofacial Surgery, University Hospital Regensburg, Regensburg, Germany"
}
],
"authenticated-orcid": false,
"family": "Gottsauner",
"given": "J.M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Otorhinolaryngology, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Vielsmeier",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Medical Microbiology and Hygiene, University of Regensburg, Regensburg, Germany"
},
{
"name": "Institute of Clinical Microbiology and Hygiene, University Hospital Regensburg, Regensburg, Germany"
}
],
"family": "Schmidt",
"given": "B.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1750-7380",
"affiliation": [
{
"name": "Department of Conservative Dentistry and Periodontology, University Hospital Regensburg, Regensburg, Germany"
}
],
"authenticated-orcid": false,
"family": "Cieplik",
"given": "F.",
"sequence": "additional"
}
],
"container-title": "Journal of Dental Research",
"container-title-short": "J Dent Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T07:10:17Z",
"timestamp": 1679382617000
},
"deposited": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T13:57:46Z",
"timestamp": 1740837466000
},
"funder": [
{
"name": "Dentaid S.L., Barcelona, Spain"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
4
]
],
"date-time": "2025-04-04T08:45:15Z",
"timestamp": 1743756315361,
"version": "3.38.0"
},
"is-referenced-by-count": 15,
"issue": "6",
"issued": {
"date-parts": [
[
2023,
3,
21
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2023,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T00:00:00Z",
"timestamp": 1679356800000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00220345231156415",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/00220345231156415",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00220345231156415",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "608-615",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
21
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1177/00220345221102310",
"doi-asserted-by": "publisher",
"key": "bibr1-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2021.03.021",
"doi-asserted-by": "publisher",
"key": "bibr2-00220345231156415"
},
{
"DOI": "10.3390/antibiotics11050688",
"doi-asserted-by": "publisher",
"key": "bibr3-00220345231156415"
},
{
"DOI": "10.1080/22221751.2022.2098059",
"doi-asserted-by": "publisher",
"key": "bibr4-00220345231156415"
},
{
"DOI": "10.1111/jopr.13220",
"doi-asserted-by": "publisher",
"key": "bibr5-00220345231156415"
},
{
"DOI": "10.1093/infdis/jiab415",
"doi-asserted-by": "publisher",
"key": "bibr6-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2021.05.021",
"doi-asserted-by": "publisher",
"key": "bibr7-00220345231156415"
},
{
"DOI": "10.1177/00220345221110444",
"doi-asserted-by": "publisher",
"key": "bibr8-00220345231156415"
},
{
"DOI": "10.3389/fmicb.2019.00587",
"doi-asserted-by": "publisher",
"key": "bibr9-00220345231156415"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "bibr10-00220345231156415"
},
{
"author": "Domênico MBD",
"journal-title": "Epidemiol Health",
"key": "bibr11-00220345231156415",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"doi-asserted-by": "publisher",
"key": "bibr12-00220345231156415"
},
{
"DOI": "10.1093/cid/ciaa1616",
"doi-asserted-by": "publisher",
"key": "bibr13-00220345231156415"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "bibr14-00220345231156415"
},
{
"DOI": "10.1016/S2213-2600(22)00226-0",
"doi-asserted-by": "publisher",
"key": "bibr15-00220345231156415"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"doi-asserted-by": "publisher",
"key": "bibr16-00220345231156415"
},
{
"DOI": "10.1002/jmv.26954",
"doi-asserted-by": "publisher",
"key": "bibr17-00220345231156415"
},
{
"DOI": "10.1111/prd.12361",
"doi-asserted-by": "publisher",
"key": "bibr18-00220345231156415"
},
{
"DOI": "10.20411/pai.v6i1.441",
"doi-asserted-by": "publisher",
"key": "bibr19-00220345231156415"
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "publisher",
"key": "bibr20-00220345231156415"
},
{
"DOI": "10.1177/0022034520943574",
"doi-asserted-by": "publisher",
"key": "bibr21-00220345231156415"
},
{
"DOI": "10.1128/AAC.00576-20",
"doi-asserted-by": "publisher",
"key": "bibr22-00220345231156415"
},
{
"DOI": "10.3389/fmicb.2022.934525",
"doi-asserted-by": "publisher",
"key": "bibr23-00220345231156415"
},
{
"DOI": "10.1016/j.adaj.2019.06.024",
"doi-asserted-by": "publisher",
"key": "bibr24-00220345231156415"
},
{
"DOI": "10.1093/infdis/jiaa471",
"doi-asserted-by": "publisher",
"key": "bibr25-00220345231156415"
},
{
"DOI": "10.1016/j.virusres.2022.198791",
"doi-asserted-by": "publisher",
"key": "bibr26-00220345231156415"
},
{
"DOI": "10.1177/0022034520914246",
"doi-asserted-by": "publisher",
"key": "bibr27-00220345231156415"
},
{
"DOI": "10.1007/s00784-021-04363-z",
"doi-asserted-by": "publisher",
"key": "bibr28-00220345231156415"
},
{
"DOI": "10.1016/S1473-3099(21)00472-2",
"doi-asserted-by": "publisher",
"key": "bibr29-00220345231156415"
},
{
"DOI": "10.3390/microorganisms10030561",
"doi-asserted-by": "publisher",
"key": "bibr30-00220345231156415"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "bibr31-00220345231156415"
},
{
"DOI": "10.1016/S2468-2667(20)30164-X",
"doi-asserted-by": "publisher",
"key": "bibr32-00220345231156415"
},
{
"DOI": "10.1038/s41368-020-0075-9",
"doi-asserted-by": "publisher",
"key": "bibr33-00220345231156415"
},
{
"DOI": "10.1177/2380084421993099",
"doi-asserted-by": "publisher",
"key": "bibr34-00220345231156415"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "publisher",
"key": "bibr35-00220345231156415"
},
{
"DOI": "10.1111/imr.13089",
"doi-asserted-by": "publisher",
"key": "bibr36-00220345231156415"
},
{
"DOI": "10.1056/NEJMc2004973",
"doi-asserted-by": "publisher",
"key": "bibr37-00220345231156415"
},
{
"DOI": "10.1038/s41598-019-44822-y",
"doi-asserted-by": "publisher",
"key": "bibr38-00220345231156415"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "bibr39-00220345231156415"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "bibr40-00220345231156415"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/00220345231156415"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of a Mouthwash Containing CHX and CPC in SARS-CoV-2–Positive Patients: A Randomized Controlled Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1177/sage-journals-update-policy",
"volume": "102"
}
