Efficacy of oral amantadine among patients hospitalised with COVID-19: A randomised, double-blind, placebo-controlled, multicentre study
et al., Respiratory Medicine, doi:10.1016/j.rmed.2023.107198, NCT04952519, Jun 2023
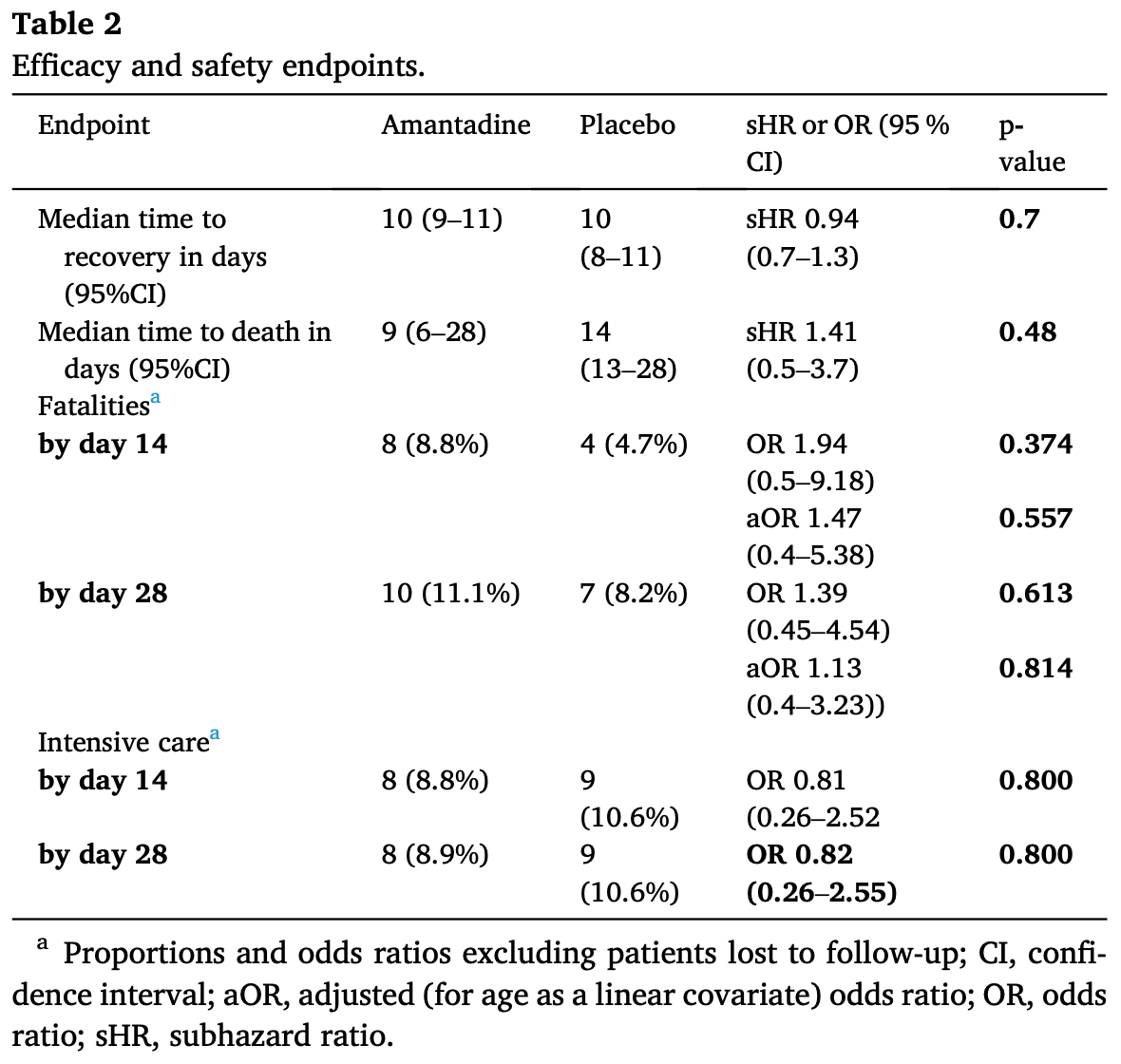
RCT 186 hospitalized COVID-19 patients showing no significant differences with oral amantadine compared to placebo.
|
risk of death, 13.0% higher, OR 1.13, p = 0.83, treatment 95, control 91, adjusted per study, RR approximated with OR.
|
|
risk of ICU admission, 18.0% lower, OR 0.82, p = 0.75, treatment 95, control 91, adjusted per study, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Barczyk et al., 30 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Poland, peer-reviewed, mean age 60.0, 20 authors, study period April 2021 - February 2022, trial NCT04952519 (history).
Contact: abarczyk@sum.edu.pl.
Efficacy of oral amantadine among patients hospitalised with COVID-19: A randomised, double-blind, placebo-controlled, multicentre study
Respiratory Medicine, doi:10.1016/j.rmed.2023.107198
Background: Amantadine has been proposed as a treatment for COVID-19 because it shows anti-SARS-CoV-2 activity in vitro. However, to date, no controlled study has assessed the safety and efficacy of amantadine in COVID-19. Research question: Whether amantadine is effective and safe among patients with different COVID-19 severity classifications. Study design: and Methods: This was multi-centre, randomised, placebo-controlled study.Patients with oxygen saturation ≤94% and no need for high-flow oxygen or ventilatory support were randomly allocated to receive oral amantadine or placebo (1:1) for 10 days in addition to standard care. The primary endpoint was time to recovery assessed over 28 days since randomisation, defined as discharge from hospital or no need for supplemental oxygen. Results: The study was terminated early due to a lack of efficacy after an interim analysis. Final data from 95 patients who received amantadine (mean age, 60.2 years; 65% male; 66% with comorbidities) and 91 patients
Methodology, Formal analysis, Investigation, Supervision, Project administration. Maciej Dyrbuś: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Rafał Harat: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Maciej Huk: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Sylwia Kotecka: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Artur Nahorecki: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Jacek Nasiłowski: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Wojciech Naumnik: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Grzegorz Przybylski: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Monika Słaboń-Willand: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Szymon Skoczyński: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Krystian Wita: Conceptualization, Methodology, Formal analysis, Supervision, Project administration, Funding acquisition. Grzegorz Zioło: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Piotr Kuna: Methodology, Formal analysis,..
References
Aoki, Sitar, Amantadine kinetics in healthy elderly men: implications for influenza prevention, Clin Pharmacol Ther [Internet, doi:10.1038/clpt.1985.25
Aranda-Abreu, Aranda-Martínez, Araújo, Hernández-Aguilar, Herrera-Covarrubias et al., Observational study of people infected with SARS-Cov-2, treated with amantadine, Pharmacol. Rep, doi:10.1007/s43440-020-00168-1
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med [Internet, doi:10.1056/NEJMoa2007764
Bodnar, Aranda-Abreu, Slabon-Willand, Kotecka, Farnik et al., The efficacy of amantadine hydrochloride in the treatment of COVID-19 -a singlecenter observation study, Pol Merkur Lekarski [Internet
Butterworth, Potential for the repurposing of adamantane antivirals for COVID-19, Drugs R, doi:10.1007/s40268-021-00351-6
Fink, Nitsche, Neumann, Grossegesse, Eisele et al., Amantadine inhibits SARS-CoV-2 in vitro, Viruses
Giacino, Whyte, Bagiella, Placebo-controlled trial of amantadine for severe traumatic brain injury, N Engl J Med [Internet, doi:10.1056/NEJMoa1102609
Hay, Wolstenholme, Skehel, Smith, The molecular basis of the specific anti-influenza action of amantadine, EMBO J [Internet
Jiménez-Jiménez, Alonso-Navarro, García-Martín, Agúndez, Antiinflammatory effects of amantadine and memantine: possible therapeutics for the treatment of Covid-19?, J. Personalized Med
Kamel, Kamel, Alhasawi, Elmasry, Alhamdan et al., Effect of pre-exposure use of amantadine on COVID-19 infection: a hospital-based Cohort study in patients with Parkinson's disease or multiple sclerosis, Front Neurol, doi:10.3389/fneur.2021.704186/full
Karlsen, Wiberg, Laigaard, Pedersen, Rokamp et al., A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment, PLoS One, doi:10.1371/journal.pone.0237903
Kim, Garg, O'halloran, Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET), Clin Infect Dis [Internet
Mancilla-Galindo, García-Méndez, Márquez-Sánchez, All-cause Mortality Among Patients Treated with Repurposed Antivirals and Antibiotics for COVID-19 in Mexico City, A Real-World Observational Study
Pucci, Brañas Tato, D'amico, Giuliani, Solari et al., Amantadine for Fatigue in Multiple Sclerosis, Cochrane Database Syst Rev [Internet, doi:10.1002/14651858.CD002818.pub2
Rascol, Fabbri, Poewe, Amantadine in the treatment of Parkinson's disease and other movement disorders, Lancet Neurol [Internet
Rejdak, Grieb, Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment, Mult Scler Relat Disord
Toft-Bertelsen, Jeppesen, Tzortzini, Amantadine inhibits known and novel ion channels encoded by SARS-CoV-2 in vitro, Commun Biol [Internet
DOI record:
{
"DOI": "10.1016/j.rmed.2023.107198",
"ISSN": [
"0954-6111"
],
"URL": "http://dx.doi.org/10.1016/j.rmed.2023.107198",
"alternative-id": [
"S0954611123000860"
],
"article-number": "107198",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy of oral amantadine among patients hospitalised with COVID-19: A randomised, double-blind, placebo-controlled, multicentre study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.rmed.2023.107198"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-6567-9208",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barczyk",
"given": "Adam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Czajkowska-Malinowska",
"given": "Małgorzata",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6090-7718",
"affiliation": [],
"authenticated-orcid": false,
"family": "Farnik",
"given": "Małgorzata",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barczyk",
"given": "Marek",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4684-5191",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boda",
"given": "Łukasz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cofta",
"given": "Szczepan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duława",
"given": "Jan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2506-2430",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dyrbuś",
"given": "Maciej",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harat",
"given": "Rafał",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0430-7141",
"affiliation": [],
"authenticated-orcid": false,
"family": "Huk",
"given": "Maciej",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kotecka",
"given": "Sylwia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nahorecki",
"given": "Artur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nasiłowski",
"given": "Jacek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naumnik",
"given": "Wojciech",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8324-163X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Przybylski",
"given": "Grzegorz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Słaboń-Willand",
"given": "Monika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Skoczyński",
"given": "Szymon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wita",
"given": "Krystian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zioło",
"given": "Grzegorz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuna",
"given": "Piotr",
"sequence": "additional"
}
],
"container-title": "Respiratory Medicine",
"container-title-short": "Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"resmedjournal.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T10:17:43Z",
"timestamp": 1678875463000
},
"deposited": {
"date-parts": [
[
2023,
4,
27
]
],
"date-time": "2023-04-27T20:09:00Z",
"timestamp": 1682626140000
},
"indexed": {
"date-parts": [
[
2025,
5,
13
]
],
"date-time": "2025-05-13T21:04:44Z",
"timestamp": 1747170284194,
"version": "3.40.5"
},
"is-referenced-by-count": 5,
"issued": {
"date-parts": [
[
2023,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0954611123000860?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0954611123000860?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "107198",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment",
"author": "Karlsen",
"issue": "8",
"journal-title": "PLoS One [Internet",
"key": "10.1016/j.rmed.2023.107198_bib1",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1002/j.1460-2075.1985.tb04038.x",
"article-title": "The molecular basis of the specific anti-influenza action of amantadine",
"author": "Hay",
"doi-asserted-by": "crossref",
"first-page": "3021",
"issue": "11",
"journal-title": "EMBO J [Internet",
"key": "10.1016/j.rmed.2023.107198_bib3",
"volume": "4",
"year": "1985"
},
{
"DOI": "10.1016/S1474-4422(21)00249-0",
"article-title": "Amantadine in the treatment of Parkinson's disease and other movement disorders",
"author": "Rascol",
"doi-asserted-by": "crossref",
"first-page": "1048",
"issue": "12",
"journal-title": "Lancet Neurol [Internet",
"key": "10.1016/j.rmed.2023.107198_bib4",
"volume": "20",
"year": "2021"
},
{
"author": "Pucci",
"key": "10.1016/j.rmed.2023.107198_bib5",
"series-title": "Amantadine for Fatigue in Multiple Sclerosis",
"year": "2007"
},
{
"DOI": "10.1038/s42003-021-02866-9",
"article-title": "Amantadine inhibits known and novel ion channels encoded by SARS-CoV-2 in vitro",
"author": "Toft-Bertelsen",
"doi-asserted-by": "crossref",
"first-page": "1347",
"issue": "1",
"journal-title": "Commun Biol [Internet",
"key": "10.1016/j.rmed.2023.107198_bib6",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.3390/v13040539",
"article-title": "Amantadine inhibits SARS-CoV-2 in vitro",
"author": "Fink",
"doi-asserted-by": "crossref",
"first-page": "539",
"issue": "4",
"journal-title": "Viruses",
"key": "10.1016/j.rmed.2023.107198_bib7",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s40268-021-00351-6",
"article-title": "Potential for the repurposing of adamantane antivirals for COVID-19",
"author": "Butterworth",
"doi-asserted-by": "crossref",
"first-page": "267",
"issue": "3",
"journal-title": "Drugs R",
"key": "10.1016/j.rmed.2023.107198_bib8",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3390/jpm10040217",
"article-title": "Anti-inflammatory effects of amantadine and memantine: possible therapeutics for the treatment of Covid-19?",
"author": "Jiménez-Jiménez",
"doi-asserted-by": "crossref",
"first-page": "217",
"issue": "4",
"journal-title": "J. Personalized Med.",
"key": "10.1016/j.rmed.2023.107198_bib9",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s43440-020-00168-1",
"article-title": "Observational study of people infected with SARS-Cov-2, treated with amantadine",
"author": "Aranda-Abreu",
"doi-asserted-by": "crossref",
"first-page": "1538",
"issue": "6",
"journal-title": "Pharmacol. Rep.",
"key": "10.1016/j.rmed.2023.107198_bib10",
"volume": "72",
"year": "2020"
},
{
"article-title": "The efficacy of amantadine hydrochloride in the treatment of COVID-19 - a single-center observation study",
"author": "Bodnar",
"first-page": "389",
"issue": "294",
"journal-title": "Pol Merkur Lekarski [Internet",
"key": "10.1016/j.rmed.2023.107198_bib11",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1016/j.msard.2020.102163",
"article-title": "Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment",
"author": "Rejdak",
"doi-asserted-by": "crossref",
"journal-title": "Mult Scler Relat Disord",
"key": "10.1016/j.rmed.2023.107198_bib12",
"volume": "42",
"year": "2020"
},
{
"article-title": "Effect of pre-exposure use of amantadine on COVID-19 infection: a hospital-based Cohort study in patients with Parkinson's disease or multiple sclerosis",
"author": "Kamel",
"journal-title": "Front Neurol [Internet",
"key": "10.1016/j.rmed.2023.107198_bib13",
"volume": "12",
"year": "2021"
},
{
"author": "Mancilla-Galindo",
"first-page": "199",
"key": "10.1016/j.rmed.2023.107198_bib14",
"volume": "vol. 20",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 — final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med [Internet",
"key": "10.1016/j.rmed.2023.107198_bib15",
"volume": "383",
"year": "2020"
},
{
"article-title": "Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET)",
"author": "Kim",
"issue": "9",
"journal-title": "Clin Infect Dis [Internet",
"key": "10.1016/j.rmed.2023.107198_bib17",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa1102609",
"article-title": "Placebo-controlled trial of amantadine for severe traumatic brain injury",
"author": "Giacino",
"doi-asserted-by": "crossref",
"first-page": "819",
"issue": "9",
"journal-title": "N Engl J Med [Internet",
"key": "10.1016/j.rmed.2023.107198_bib18",
"volume": "366",
"year": "2012"
},
{
"DOI": "10.1038/clpt.1985.25",
"article-title": "Amantadine kinetics in healthy elderly men: implications for influenza prevention",
"author": "Aoki",
"doi-asserted-by": "crossref",
"first-page": "137",
"issue": "2",
"journal-title": "Clin Pharmacol Ther [Internet",
"key": "10.1016/j.rmed.2023.107198_bib19",
"volume": "37",
"year": "1985"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0954611123000860"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Efficacy of oral amantadine among patients hospitalised with COVID-19: A randomised, double-blind, placebo-controlled, multicentre study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "212"
}