Various Combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, High-Dose Oseltamivir, and Hydroxychloroquine for the Treatment of COVID-19: A Randomized Controlled Trial (FIGHT-COVID-19 Study)
et al., SSRN Electronic Journal, doi:10.2139/ssrn.3936499, NCT04303299, Oct 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 320 patients in Thailand, showing significantly lower progression with HCQ for moderate/severe patients, and faster viral clearance with mild patients (statistically significant for 800mg). There are two sets of results - for moderate/severe patients, and for mild patients. There was no mortality for mild patients. NCT04303299 (history).
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Study covers favipiravir and HCQ.
|
risk of death, 56.2% lower, RR 0.44, p = 0.07, treatment 7 of 100 (7.0%), control 16 of 100 (16.0%), NNT 11, moderate/severe, HCQ arms vs. non-HCQ arms.
|
|
risk of progression, 54.2% lower, RR 0.46, p = 0.02, treatment 11 of 100 (11.0%), control 24 of 100 (24.0%), NNT 7.7, moderate/severe, HCQ arms vs. non-HCQ arms.
|
|
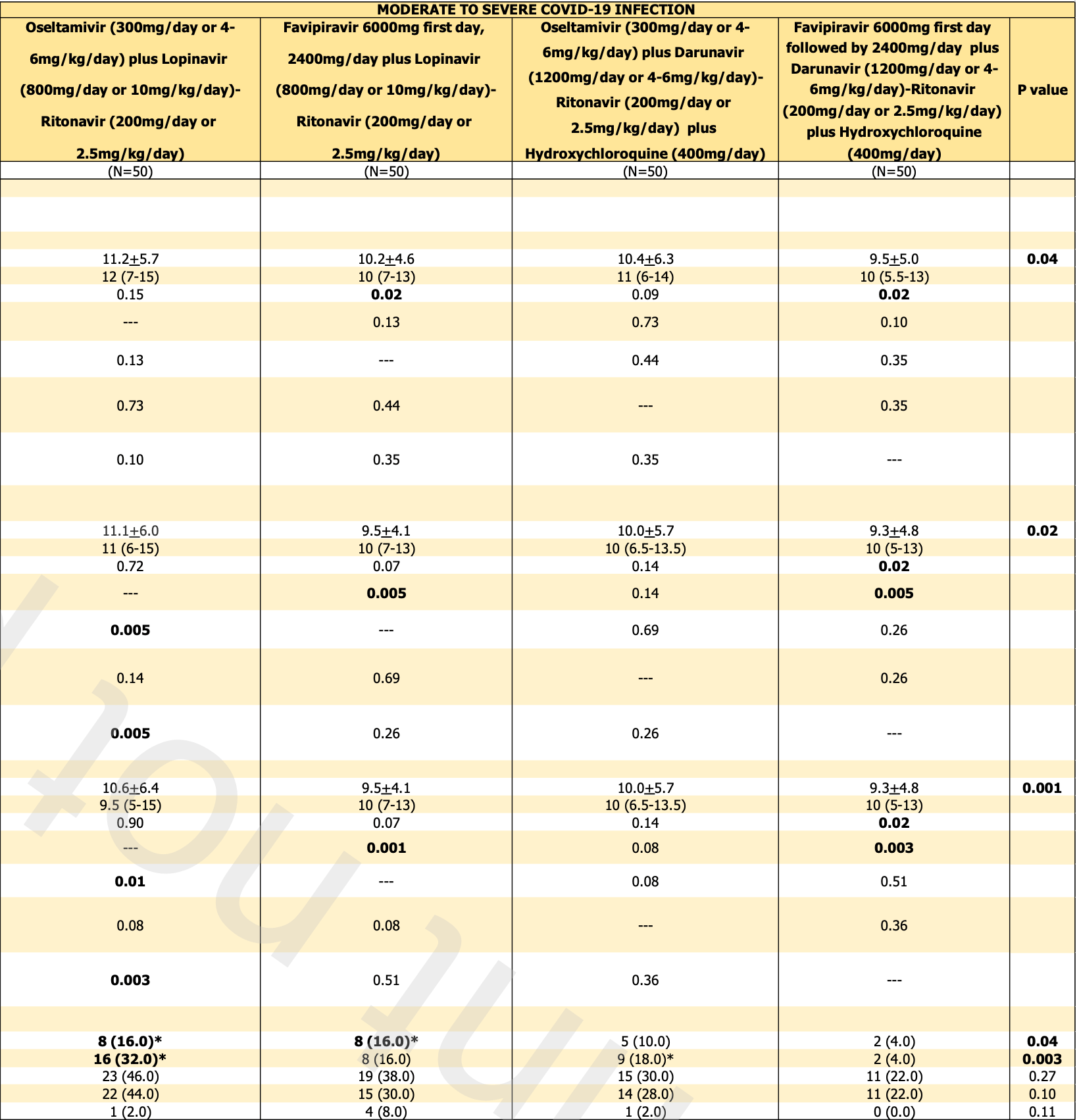
time to viral-, 7.1% lower, relative time 0.93, p = 0.51, treatment mean 10.4 (±6.3) n=50, control mean 11.2 (±5.7) n=50, moderate/severe, oseltamivir arms, primary outcome.
|
|
time to viral-, 6.9% lower, relative time 0.93, p = 0.47, treatment mean 9.5 (±5.0) n=50, control mean 10.2 (±4.6) n=50, moderate/severe, favipiravir arms, primary outcome.
|
|
risk of progression, 150.0% higher, RR 2.50, p = 1.00, treatment 1 of 60 (1.7%), control 0 of 30 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), mild, early treatment result.
|
|
time to viral-, 43.3% lower, relative time 0.57, p = 0.04, treatment mean 8.9 (±6.0) n=30, control mean 15.7 (±16.7) n=30, mild, HCQ 800, primary outcome, early treatment result.
|
|
time to viral-, 36.3% lower, relative time 0.64, p = 0.09, treatment mean 10.0 (±6.9) n=30, control mean 15.7 (±16.7) n=30, mild, HCQ 400, primary outcome, early treatment result.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Atipornwanich et al., 5 Oct 2021, Randomized Controlled Trial, Thailand, peer-reviewed, 16 authors, study period 19 October, 2020 - 20 July, 2021, dosage 400mg days 1-14, 800mg/day or 400mg/day, this trial compares with another treatment - results may be better when compared to placebo, this trial uses multiple treatments in the treatment arm (combined with oseltamivir/favipiravir and duranivir/ritonavir for moderate/severe, oseltamivir and duranivir/ritonavir for mild) - results of individual treatments may vary, trial NCT04303299 (history).
Various combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, high-dose Oseltamivir, and Hydroxychloroquine for the treatment of Covid-19: A randomized controlled trial. (FIGHT-COVID-19 Study)
References
Hata, Koseki, Yamaguchi, Limited inhibitory effects of Oseltamivir and zanamivir on human sialidases, Antimicrob Agents Chemother, doi:10.1128/AAC.00344-08
Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavirritonavir, and ribavirin in the treatment of patients admitted to hospital with Covid -19: an open-label, randomised, phase 2 trial, Lancet, doi:10.1016/S0140-6736(20)31042-4
Kriangsak, Akksilp, Sawanpanyalert, Srisubat, Thanasithichai et al., Various Combination of Antiviral Treatment of Covid -19 Pneumonia
Shinkai, Tsushima, Tanaka, A Randomized, Phase III Clinical Trial, Infect Dis Ther, doi:10.1007/s40121-021-00517-4
Wang, Zhang, Du, Remdesivir in adults with severe Covid -19: a randomised, doubleblind, placebo-controlled, multicentre trial [published correction appears in, Lancet
DOI record:
{
"DOI": "10.2139/ssrn.3936499",
"ISSN": [
"1556-5068"
],
"URL": "http://dx.doi.org/10.2139/ssrn.3936499",
"author": [
{
"affiliation": [],
"family": "Atipornwanich",
"given": "Kriangsak",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kongsaengdao",
"given": "Subsai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harnsomburana",
"given": "Piyathida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nanna",
"given": "Rienthong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chtuparisute",
"given": "Chatchawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saengsayan",
"given": "Piamlarp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bangpattanasiri",
"given": "Kittima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manosuthi",
"given": "Weerawat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sawanpanyalert",
"given": "Narumol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Srisubat",
"given": "Attasit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thanasithichai",
"given": "Somchai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maneeton",
"given": "Benchalak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maneeton",
"given": "Narong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suthisisang",
"given": "Chuthamanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pratuangdejkul",
"given": "Jaturong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akksilp",
"given": "Somsak",
"sequence": "additional"
}
],
"container-title": [
"SSRN Electronic Journal"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
10
]
],
"date-time": "2021-10-10T12:55:10Z",
"timestamp": 1633870510000
},
"deposited": {
"date-parts": [
[
2021,
10,
10
]
],
"date-time": "2021-10-10T12:55:13Z",
"timestamp": 1633870513000
},
"indexed": {
"date-parts": [
[
2022,
1,
10
]
],
"date-time": "2022-01-10T12:29:11Z",
"timestamp": 1641817751857
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "electronic",
"value": "1556-5068"
}
],
"issued": {
"date-parts": [
[
2021
]
]
},
"language": "en",
"member": "78",
"original-title": [],
"prefix": "10.2139",
"published": {
"date-parts": [
[
2021
]
]
},
"published-other": {
"date-parts": [
[
2021
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"SSRN Journal"
],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": [
"Various Combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, High-Dose Oseltamivir, and Hydroxychloroquine for the Treatment of COVID-19: A Randomized Controlled Trial (FIGHT-COVID-19 Study)"
],
"type": "journal-article"
}
