Previous Vitamin D Supplementation and Morbidity and Mortality Outcomes in People Hospitalised for COVID19: A Cross-Sectional Study
et al., Frontiers in Public Health, doi:10.3389/fpubh.2021.758347, Sep 2021
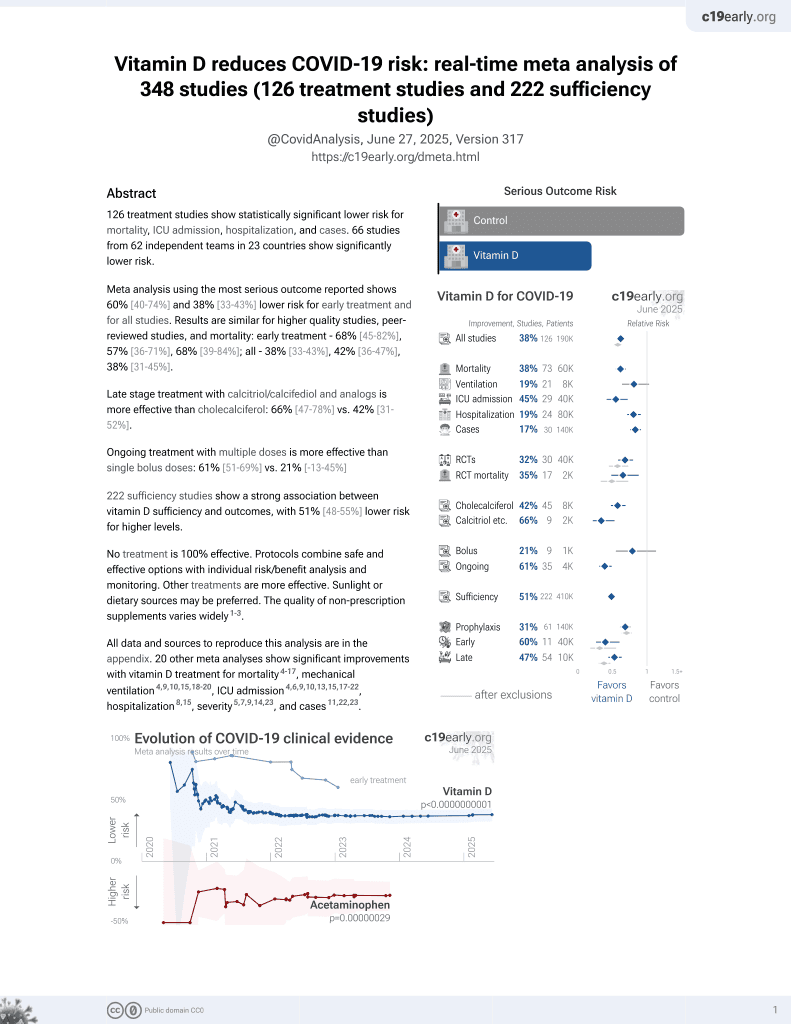
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 1,267 hospitalized patients in Spain, 189 on vitamin D supplementation before admission, showing lower ICU admission with supplementation, and no statistically significant difference for mortality or ventilation.
This is the 55th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
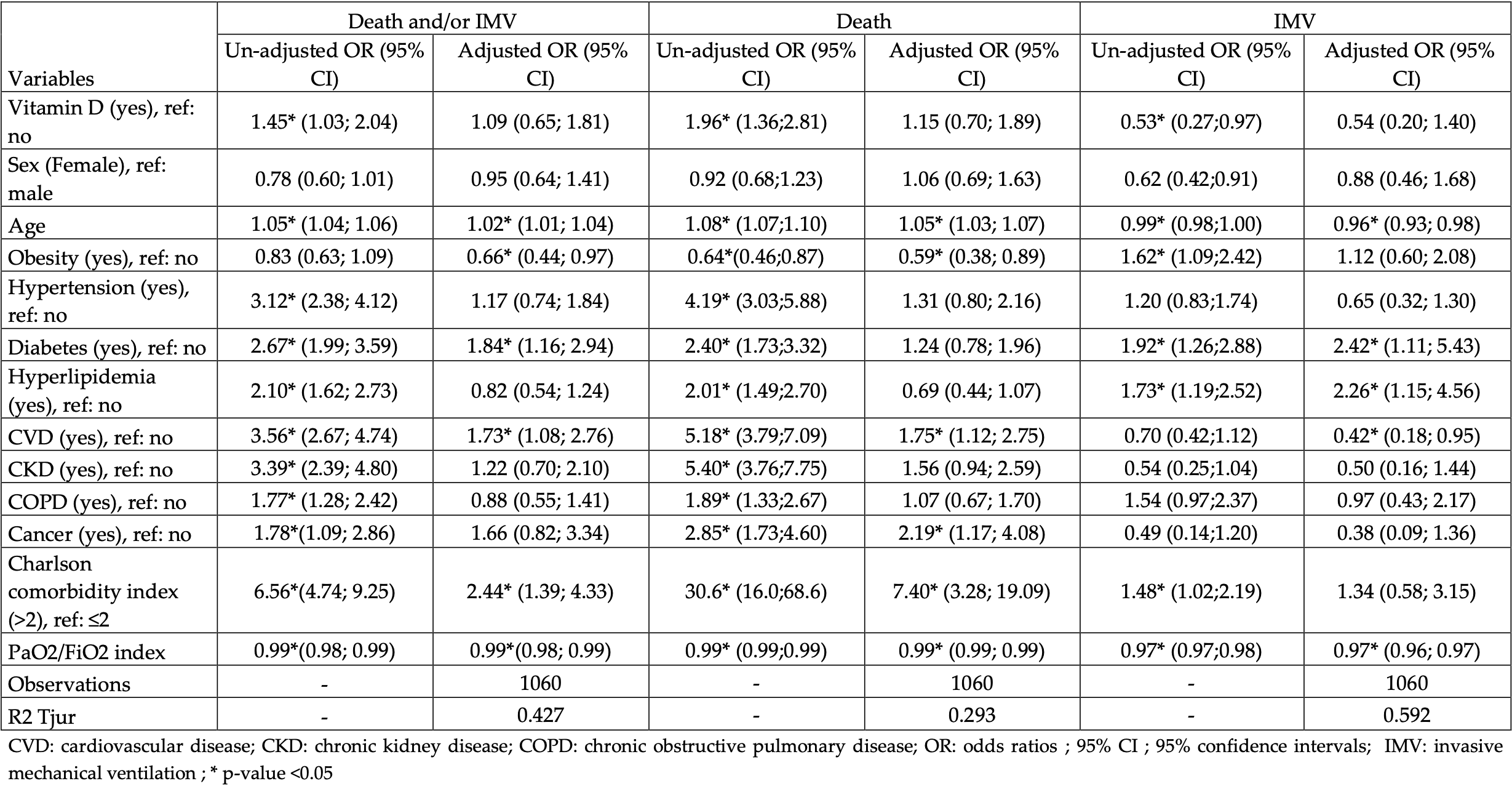
risk of death, 12.4% higher, RR 1.12, p = 0.59, treatment 50 of 189 (26.5%), control 167 of 1,078 (15.5%), adjusted per study, odds ratio converted to relative risk.
|
|
risk of mechanical ventilation, 43.3% lower, RR 0.57, p = 0.22, treatment 11 of 189 (5.8%), control 113 of 1,078 (10.5%), NNT 21, adjusted per study, odds ratio converted to relative risk.
|
|
risk of ICU admission, 44.2% lower, RR 0.56, p = 0.03, treatment 13 of 189 (6.9%), control 133 of 1,078 (12.3%), NNT 18, unadjusted.
|
|
hospitalization time, 11.8% lower, relative time 0.88, p = 0.20, treatment 189, control 1,078, unadjusted.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Arroyo-Díaz et al., 24 Sep 2021, retrospective, Spain, peer-reviewed, 11 authors, dosage not specified.
Previous Vitamin D Supplementation and Morbidity and Mortality Outcomes in People Hospitalised for COVID19: A Cross-Sectional Study
Frontiers in Public Health, doi:10.3389/fpubh.2021.758347
Aim: The study aim was to assess the association of vitamin D supplementation before hospital admission and severe outcomes in subjects admitted for COVID-19. Methods: We performed a cross-sectional analysis of pseudonymised medical record data from subjects admitted to the Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) for COVID-19 during March and April 2020. The composite primary study outcome was defined as death and/or invasive mechanical ventilation (IMV). Association between risk factors and study outcomes was evaluated by bivariate analysis, followed by logistic regression analysis. Results: In total, 1,267 persons were hospitalised during the observation period. Overall, 14.9% of the subjects were on active vitamin D supplementation treatment before admission. The subjects in the vitamin D group were significantly older than subjects without vitamin D supplementation. We observed higher rates of the primary outcome (death and/or IMV) among the persons with previous use of vitamin D (30.1 vs. 22.9% in those not receiving treatment). In the bivariate analysis, previous use of vitamin D was positively associated with death and/or IMV [odds ratio (OR): 1.45 95% CI: 1.03; 2.04]; however, after adjustment for other risk factors this association disappeared (OR: 1.09 95%CI: 0.65; 1.81). Arroyo-Díaz et al. Vitamin D Supplementation and Severity of COVID19 Conclusion: We did not find an association between vitamin D supplementation before hospital admission and death and/or IMV in subjects admitted for COVID-19. The age and the burden of age-associated comorbidities were independently associated with the in-hospital events.
DATA AVAILABILITY STATEMENT The data analysed in this study is subject to the following licences/restrictions: The data controller for Hospital de la Santa Creu i Sant Pau does not allow the sharing of raw data. Requests to access these datasets should be directed to Pere Domingo, pdomingo@santpau.cat.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by the Ethics Committee of the Hospital de la Santa Creu i Sant Pau (Re. Nr. HSCSP-20/117). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AUTHOR CONTRIBUTIONS JA-D, JJ, JF-N, PD, and DM: conceptualisation. EN-M: formal analysis. JA-D, DM, PD, ER, and PP: resources and data curation. BV: writing-original draught preparation. BV, RC, JA-D, GL, JF-N, PD, and DM: writing-review and editing. DM and JF-N: supervision. JA-D: project administration. All authors contributed to the article and approved the submitted version.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh. 2021.758347/full#supplementary-material Conflict of Interest: RC has received advisory and/or speaking fees from Abbott, Ascensia, Lilly, MSD, Novo and Sanofi. JF-N has received advisory and or speaking fees from Astra-Zeneca, Ascensia, Boehringer Ingelheim, GSK, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi; they received..
References
Annweiler, Corvaisier, Gautier, Dubée, Legrand et al., Vitamin d supplementation associated to better survival in hospitalised frail elderly covid-19 subjects: the geria-covid quasi-experimental study, Nutrients, doi:10.3390/nu12113377
Annweiler, Hanotte, De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 subjects: a quasiexperimental study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105771
Bassatne, Basbous, Chakhtoura, El Zein, Rahme et al., The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis, Metabolism, doi:10.1016/j.metabol.2021.154753
Caballero, Guide to Nutritional Supplements
Casado, Quesada, Naves, Peris, Jódar et al., Recomendaciones de la SEIOMM en la prevención y tratamiento del déficit de vitamina D, Rev Osteoporos Metab Min, doi:10.4321/S1889-836X2021000200007
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among subjects hospitalised for COVID-19: a pilot randomised clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Cozier, Castro-Webb, Hochberg, Rosenberg, Palmer, Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in US Black women, PLoS ONE, doi:10.1371/journal.pone.0255132
Crafa, Cannarella, Condorelli, Mongioì, Barbagallo et al., Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: a systematic review and metaanalysis, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100967
Dana, Bannay, Bourst, Ziegler, Losser et al., Obesity and mortality in critically ill COVID-19 patients with respiratory failure, Int J Obes, doi:10.1038/s41366-021-00872-9
Dramé, Cofais, Hentzien, Proye, Coulibaly et al., Relation between vitamin D and covid-19 in aged people: a systematic review, Nutrients, doi:10.3390/nu13041339
González-Molero, Morcillo, Valdés, Pérez-Valero, Botas et al., Vitamin D deficiency in Spain: a population-based cohort study, Eur J Clin Nutr, doi:10.1038/ejcn.2010.265
Grove, Osokogu, Al-Khudairy, Mehrabian, Zanganeh et al., Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review, BMJ Open, doi:10.1136/bmjopen-2020-043737
Gu, Wang, Chen, Lu, Liu et al., PaO 2 /FiO 2 and IL-6 are risk factors of mortality for intensive care COVID-19 subjects, Sci Rep, doi:10.1038/s41598-021-86676-3
Gunville, Mourani, Ginde, The role of vitamin D in prevention and treatment of infection, Inflamm Allergy-Drug Targets, doi:10.2174/18715281113129990046
Hernández, Nan, Fernandez-Ayala, García-Unzueta, Hernández-Hernández et al., Vitamin D status in hospitalized subjects with SARS-CoV-2 infection, J Clin Endocrinol Metab, doi:10.1210/clinem/dgaa733
Ingham, Jones, Camargo, Kirman, Dowell et al., Association of vitamin D deficiency with severity of acute respiratory infection: A CASE-control study in New Zealand children, Eur Respir J
Karahan, Katkat, Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey, J Nutr Health Aging, doi:10.1007/s12603-020-1479-0
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS ONE, doi:10.1371/journal.pone.0239252
Kazemi, Mohammadi, Aghababaee, Golzarand, Clark et al., Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis, Adv Nutr, doi:10.1093/advances/nmab012
Liu, Mao, Liang, Yang, Lu et al., Association between age and clinical characteristics and outcomes of COVID-19, Eur Respir J, doi:10.1183/13993003.01112-2020
Macaya, Paeres, Valls, Fernández-Ortiz, Del Castillo et al., Interaction between age and vitamin D deficiency in severe COVID-19 infection, Nutr Hosp, doi:10.20960/nh.03193
Mahase, Covid-19: death rate is 0.66% and increases with age, study estimates, BMJ, doi:10.1136/bmj.m1327
Meehan, Penckofer, The role of vitamin D in the aging adult, J Aging Gerontol, doi:10.12974/2309-6128.2014.02.02.1
Meltzer, Best, Zhang, Vokes, Arora et al., Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.4117
Meltzer, Best, Zhang, Vokes, Arora et al., Association of vitamin D status and other clinical characteristics with COVID-19 test results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.19722
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of vitamin D3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: a multicenter, double-blind, randomized controlled trial, doi:10.1101/2020.11.16.20232397
Oscanoa, Amado, Vidal, Laird, Ghashut et al., The relationship between the severity and mortality of SARS-COV-2 infection and 25-hydroxy vitamin D concentration -a meta-analysis, Adv Respir Med, doi:10.5603/ARM.a2021.0037
Petrelli, Luciani, Perego, Dognini, Colombelli et al., Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2021.105883
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger, Vitamin D deficiency and outcome of COVID-19 subjects, Nutrients, doi:10.3390/nu12092757
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Sabetta, Depetrillo, Cipriani, Smardin, Burns et al., Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults, PLoS ONE, doi:10.1371/journal.pone.0011088
Spiro, Buttriss, Vitamin D: an overview of vitamin D status and intake in Europe, Nutr Bull, doi:10.1111/nbu.12108
Stroehlein, Wallqvist, Iannizzi, Mikolajewska, Metzendorf et al., Vitamin D supplementation for the treatment of COVID-19: a living systematic review, Cochrane Database. Syst Rev, doi:10.1002/14651858.CD015043
Verity, Okell, Dorigatti, Winskill, Whittaker et al., Estimates of the severity of coronavirus disease 2019: a model-based analysis, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30243-7
DOI record:
{
"DOI": "10.3389/fpubh.2021.758347",
"ISSN": [
"2296-2565"
],
"URL": "http://dx.doi.org/10.3389/fpubh.2021.758347",
"abstract": "<jats:p><jats:bold>Aim:</jats:bold> The study aim was to assess the association of vitamin D supplementation before hospital admission and severe outcomes in subjects admitted for COVID-19.</jats:p><jats:p><jats:bold>Methods:</jats:bold> We performed a cross-sectional analysis of pseudonymised medical record data from subjects admitted to the Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) for COVID-19 during March and April 2020. The composite primary study outcome was defined as death and/or invasive mechanical ventilation (IMV). Association between risk factors and study outcomes was evaluated by bivariate analysis, followed by logistic regression analysis.</jats:p><jats:p><jats:bold>Results:</jats:bold> In total, 1,267 persons were hospitalised during the observation period. Overall, 14.9% of the subjects were on active vitamin D supplementation treatment before admission. The subjects in the vitamin D group were significantly older than subjects without vitamin D supplementation. We observed higher rates of the primary outcome (death and/or IMV) among the persons with previous use of vitamin D (30.1 vs. 22.9% in those not receiving treatment). In the bivariate analysis, previous use of vitamin D was positively associated with death and/or IMV [odds ratio (OR): 1.45 95% CI: 1.03; 2.04]; however, after adjustment for other risk factors this association disappeared (OR: 1.09 95%CI: 0.65; 1.81).</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> We did not find an association between vitamin D supplementation before hospital admission and death and/or IMV in subjects admitted for COVID-19. The age and the burden of age-associated comorbidities were independently associated with the in-hospital events.</jats:p>",
"alternative-id": [
"10.3389/fpubh.2021.758347"
],
"author": [
{
"affiliation": [],
"family": "Arroyo-Díaz",
"given": "Juan Antonio",
"sequence": "first"
},
{
"affiliation": [],
"family": "Julve",
"given": "Josep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vlacho",
"given": "Bogdan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Corcoy",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponte",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Román",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navas-Méndez",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Llauradó",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Franch-Nadal",
"given": "Josep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Domingo",
"given": "Pere",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mauricio",
"given": "Didac",
"sequence": "additional"
}
],
"container-title": "Frontiers in Public Health",
"container-title-short": "Front. Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
9,
25
]
],
"date-time": "2021-09-25T00:41:32Z",
"timestamp": 1632530492000
},
"deposited": {
"date-parts": [
[
2021,
9,
25
]
],
"date-time": "2021-09-25T00:42:02Z",
"timestamp": 1632530522000
},
"indexed": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T15:20:28Z",
"timestamp": 1709133628928
},
"is-referenced-by-count": 8,
"issued": {
"date-parts": [
[
2021,
9,
24
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
24
]
],
"date-time": "2021-09-24T00:00:00Z",
"timestamp": 1632441600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fpubh.2021.758347/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
9,
24
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
24
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "B1",
"unstructured": "2021"
},
{
"key": "B2",
"unstructured": "COVID-19 Weekly Epidemiological Update 222021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for covid-19—interim WHO solidarity trial results",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "B3",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "B4",
"volume": "384",
"year": "2021"
},
{
"author": "Caballero",
"key": "B5",
"volume-title": "Guide to Nutritional Supplements",
"year": "2009"
},
{
"DOI": "10.1038/ejcn.2010.265",
"article-title": "Vitamin D deficiency in Spain: a population-based cohort study",
"author": "González-Molero",
"doi-asserted-by": "publisher",
"first-page": "321",
"journal-title": "Eur J Clin Nutr.",
"key": "B6",
"volume": "65",
"year": "2011"
},
{
"DOI": "10.2174/18715281113129990046",
"article-title": "The role of vitamin D in prevention and treatment of infection",
"author": "Gunville",
"doi-asserted-by": "publisher",
"first-page": "239",
"journal-title": "Inflamm Allergy-Drug Targets",
"key": "B7",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among subjects hospitalised for COVID-19: a pilot randomised clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "publisher",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "B8",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.3390/nu12113377",
"article-title": "Vitamin d supplementation associated to better survival in hospitalised frail elderly covid-19 subjects: the geria-covid quasi-experimental study",
"author": "Annweiler",
"doi-asserted-by": "publisher",
"first-page": "3377",
"journal-title": "Nutrients.",
"key": "B9",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"article-title": "Association of vitamin D status and other clinical characteristics with COVID-19 test results",
"author": "Meltzer",
"doi-asserted-by": "publisher",
"first-page": "e2019722",
"journal-title": "JAMA Netw Open",
"key": "B10",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0239252",
"article-title": "SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels",
"author": "Kaufman",
"doi-asserted-by": "publisher",
"first-page": "e0239252",
"journal-title": "PLoS ONE",
"key": "B11",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0255132",
"article-title": "Palmer JR. Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in US Black women",
"author": "Cozier",
"doi-asserted-by": "publisher",
"first-page": "e0255132",
"journal-title": "PLoS ONE.",
"key": "B12",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.4117",
"article-title": "Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results",
"author": "Meltzer",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "JAMA Netw Open.",
"key": "B13",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100967",
"article-title": "Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: a systematic review and meta-analysis",
"author": "Crafa",
"doi-asserted-by": "publisher",
"first-page": "100967",
"journal-title": "EClinicalMedicine.",
"key": "B14",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.metabol.2021.154753",
"article-title": "The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis",
"author": "Bassatne",
"doi-asserted-by": "publisher",
"first-page": "154753",
"journal-title": "Metabolism.",
"key": "B15",
"volume": "119",
"year": "2021"
},
{
"DOI": "10.1007/s12603-020-1479-0",
"article-title": "Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey",
"author": "Karahan",
"doi-asserted-by": "publisher",
"first-page": "189",
"journal-title": "J Nutr Health Aging",
"key": "B16",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1210/clinem/dgaa733",
"article-title": "Vitamin D status in hospitalized subjects with SARS-CoV-2 infection",
"author": "Hernández",
"doi-asserted-by": "publisher",
"first-page": "e1343",
"journal-title": "J Clin Endocrinol Metab.",
"key": "B17",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.3390/nu12092757",
"article-title": "Vitamin D deficiency and outcome of COVID-19 subjects",
"author": "Radujkovic",
"doi-asserted-by": "publisher",
"first-page": "2757",
"journal-title": "Nutrients.",
"key": "B18",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.20960/nh.03193",
"article-title": "Interaction between age and vitamin D deficiency in severe COVID-19 infection",
"author": "Macaya",
"doi-asserted-by": "publisher",
"first-page": "1039",
"journal-title": "Nutr Hosp",
"key": "B19",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.1093/advances/nmab012.",
"article-title": "Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis",
"author": "Kazemi",
"doi-asserted-by": "publisher",
"journal-title": "Adv Nutr",
"key": "B20",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"article-title": "Vitamin D and survival in COVID-19 subjects: a quasi-experimental study",
"author": "Annweiler",
"doi-asserted-by": "publisher",
"first-page": "105771",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "B21",
"volume": "204",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-86676-3",
"article-title": "PaO2/FiO2 and IL-6 are risk factors of mortality for intensive care COVID-19 subjects",
"author": "Gu",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Sci Rep.",
"key": "B22",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41366-021-00872-9",
"article-title": "Obesity and mortality in critically ill COVID-19 patients with respiratory failure",
"author": "Dana",
"doi-asserted-by": "publisher",
"first-page": "2028",
"journal-title": "Int J Obes",
"key": "B23",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-043737",
"article-title": "Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review",
"author": "Grove",
"doi-asserted-by": "publisher",
"first-page": "e043737",
"journal-title": "BMJ Open.",
"key": "B24",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/nu13041339",
"article-title": "Relation between vitamin D and covid-19 in aged people: a systematic review",
"author": "Dramé",
"doi-asserted-by": "publisher",
"first-page": "1339",
"journal-title": "Nutrients.",
"key": "B25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1327",
"article-title": "Covid-19: death rate is 0.66% and increases with age, study estimates",
"author": "Mahase",
"doi-asserted-by": "publisher",
"first-page": "m1327",
"journal-title": "BMJ.",
"key": "B26",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"article-title": "Estimates of the severity of coronavirus disease 2019: a model-based analysis",
"author": "Verity",
"doi-asserted-by": "publisher",
"first-page": "669",
"journal-title": "Lancet Infect Dis.",
"key": "B27",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01112-2020",
"article-title": "Association between age and clinical characteristics and outcomes of COVID-19",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "2001112",
"journal-title": "Eur Respir J.",
"key": "B28",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD015043",
"article-title": "Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database",
"author": "Stroehlein",
"doi-asserted-by": "publisher",
"first-page": "CD015043",
"journal-title": "Syst Rev.",
"key": "B29",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.5603/ARM.a2021.0037",
"article-title": "The relationship between the severity and mortality of SARS-COV-2 infection and 25-hydroxy vitamin D concentration - a meta-analysis",
"author": "Oscanoa",
"doi-asserted-by": "publisher",
"first-page": "175",
"journal-title": "Adv Respir Med.",
"key": "B30",
"volume": "89",
"year": "2021"
},
{
"DOI": "10.1101/2020.11.16.20232397",
"article-title": "Effect of vitamin D3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: a multicenter, double-blind, randomized controlled trial",
"author": "Murai",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv [preprint]",
"key": "B31",
"year": "2020"
},
{
"article-title": "Association of vitamin D deficiency with severity of acute respiratory infection: A CASE-control study in New Zealand children",
"author": "Ingham",
"first-page": "44",
"journal-title": "Eur Respir J",
"key": "B32",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0011088",
"article-title": "Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults",
"author": "Sabetta",
"doi-asserted-by": "publisher",
"first-page": "e11088",
"journal-title": "PLoS ONE",
"key": "B33",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.jsbmb.2021.105883",
"article-title": "Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies",
"author": "Petrelli",
"doi-asserted-by": "publisher",
"first-page": "105883",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "B34",
"volume": "211",
"year": "2021"
},
{
"DOI": "10.12974/2309-6128.2014.02.02.1",
"article-title": "The role of vitamin D in the aging adult",
"author": "Meehan",
"doi-asserted-by": "publisher",
"first-page": "60",
"journal-title": "J Aging Gerontol.",
"key": "B35",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1111/nbu.12108",
"article-title": "Vitamin D: an overview of vitamin D status and intake in Europe",
"author": "Spiro",
"doi-asserted-by": "publisher",
"first-page": "322",
"journal-title": "Nutr Bull.",
"key": "B36",
"volume": "39",
"year": "2014"
},
{
"DOI": "10.4321/S1889-836X2021000200007",
"article-title": "Recomendaciones de la SEIOMM en la prevención y tratamiento del déficit de vitamina D",
"author": "Casado",
"doi-asserted-by": "publisher",
"first-page": "84",
"journal-title": "Rev Osteoporos Metab Min.",
"key": "B37",
"volume": "13",
"year": "2021"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fpubh.2021.758347/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Public Health, Environmental and Occupational Health"
],
"subtitle": [],
"title": "Previous Vitamin D Supplementation and Morbidity and Mortality Outcomes in People Hospitalised for COVID19: A Cross-Sectional Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}