Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial
et al., Canadian Medical Association Journal, doi:10.1503/cmaj.211698, CATCO, NCT04330690, Jan 2022
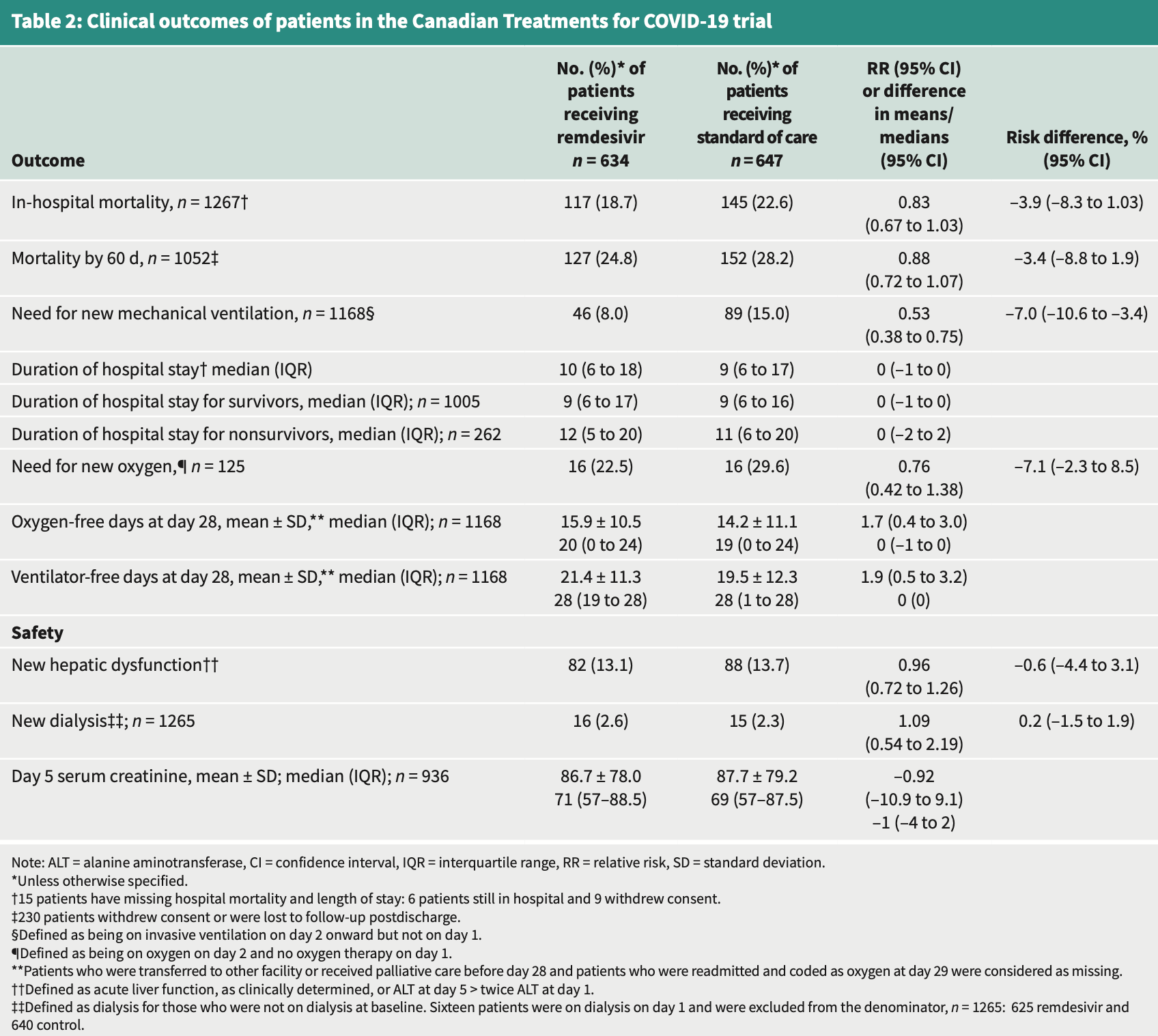
RCT 1,282 hospitalized patients in Canada showing lower mechanical ventilation with remdesivir treatment, but no significant difference for mortality.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
|
risk of death, 12.0% lower, RR 0.88, p = 0.21, treatment 127 of 634 (20.0%), control 152 of 647 (23.5%), NNT 29, day 60.
|
|
risk of death, 17.0% lower, RR 0.83, p = 0.09, treatment 117 of 634 (18.5%), control 145 of 647 (22.4%), NNT 25, in hospital.
|
|
risk of death, 20.6% lower, RR 0.79, p = 0.59, treatment 14 of 634 (2.2%), control 18 of 647 (2.8%), NNT 174, day 15.
|
|
risk of mechanical ventilation, 47.0% lower, RR 0.53, p < 0.001, treatment 46 of 634 (7.3%), control 89 of 647 (13.8%), NNT 15, day 60.
|
|
risk of no recovery, 9.0% lower, RR 0.91, p = 0.41, treatment 634, control 647, clinical status, day 60.
|
|
hospitalization time, 11.1% higher, relative time 1.11, p = 0.04, treatment median 10.0 IQR 12.0 n=634, control median 9.0 IQR 11.0 n=647.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Ali et al., 19 Jan 2022, Randomized Controlled Trial, Canada, peer-reviewed, 85 authors, average treatment delay 8.0 days, trial NCT04330690 (history) (CATCO).
Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial
Canadian Medical Association Journal, doi:10.1503/cmaj.211698
T he role of remdesivir in treating patients in hospital with COVID-19 remains ill defined. 1 Remdesivir, a repurposed antiviral medication, has full or emergency approval from a number of regulators -including Health Canada -for the treatment of COVID-19, based on clinical trial data documenting a benefit on improving time to recovery. 2 An interim report of the larger World Health Organization (WHO) Solidarity trial showed no difference regarding mortality or need for mechanical ventilation, with a number of smaller trials being inconclusive on these important outcomes. [3] [4] [5] [6] Recommendations of clinical guidelines are mixed, with some recommending remdesivir as standard of care, and others weakly recommending against. 7, 8 Its impact on other clinical outcomes, including resource utilization and post-hospital stay outcomes, has not been fully defined, and there remains a possibility of an important treatment effect, particularly in certain groups of patients. 9
Research Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial Canadian Treatments for COVID-19 (CATCO)*; for the Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group
Authors: Karim Ali, Tanweer Azher, Mahin Baqi, Alexandra Binnie, Competing interests: Alexandra Binnie reports receiving research grants from the Canadian Institutes of Health Research (CIHR) and the Physicians Services Incorporated Foundation. Sergio Borgia reports receiving honoraria from Gilead Sciences and GSK. Yiorgos Alexandros Cavayas reports receiving a grant from CIHR. Matthew Cheng reports receiving grants from the McGill Interdisciplinary Initiative in Infection and Immunity and from CIHR, during the conduct of the study (payments made to the institution). Dr. Cheng also reports receiving personal fees from AstraZeneca, outside the submitted work; and from Nplex Biosciences and GEn1E lifesciences (in the form of stock options for being a member of the scientific advisory board) outside the submitted work. Dr. Cheng co-founded Kanvas Biosicences and owns equity in the company, and reports 3 patents pending. Ameeta Singh reports receiving consulting fees from Gilead for membership of an advisory board. Ranjani Somayaji reports receiving contract research funding from Sunnybrook Research Institute, University of Calgary and Calgary Health Foundation, and clinical research funding from CIHR and the Cystic Fibrosis Foundation. Dr. Somayaji also reports participation on an oncovir data monitoring safety board. Darrell Tan reports receiving grants from AbbVie (in-kind drug only) and Gilead (inkind drug and grants to institution), and a contract between GSK and the..
References
Ader, Bouscambert-Duchamp, Hites, Discovery Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00485-0
Adhikari, Fowler, Bhagwanjee, Critical care and the global burden of critical illness in adults, Lancet
Agarwal, Rochwerg, Siemieniuk, A living WHO guideline on drugs for COVID-19, BMJ
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19: final report, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19: preliminary report. Reply, N Engl J Med
Bhimraj, Morgan, Shumaker, IDSA guidelines on the treatment and management of patients with COVID-19
Borgia, Carrier, Cavayas, Chagnon, Cheng et al., Departments of Anesthesiology (Carrier), Medicine (Duceppe, Kolan), Intensive Care Medicine (Carrier) and Internal Medicine (Duceppe, Kolan) and Internal Medicine Service
Donnelly, Wang, Iwashyna, Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system, JAMA
Estcourt, Turgeon, Mcquilten, Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA
Ford, Pragmatic trials, N Engl J Med
French, Hulse, Nguyen, Impact of hospital strain on excess deaths during the COVID-19 pandemic: United States, July 2020-July 2021, MMWR Morb Mortal Wkly Rep
Goldman, Lye, Hui, Remdesivir for 5 or 10 days in patients with severe COVID-19, N Engl J Med
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Lee, Lee, Kim, TMPRSS2 and RNA-dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID-19 caused by SARS-CoV-2 variants (B.1.1.7 and B.1.351, Microbiol Spectr
Lee, Mcdonald, Butler-Laporte, Remdesivir and systemic corticosteroids for the treatment of COVID-19: a Bayesian re-analysis, Int J Infect Dis
Mahajan, Singh, Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: a prospective randomised study, Indian J Anaesth
Murthy, Archambault, Atique, SPRINT-SARI Canada Investigators and the Canadian Critical Care Trials Group. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study, CMAJ Open
Naylor, Boozary, Adams, Canadian federal-provincial/territorial funding of universal health care: fraught history, uncertain future, CMAJ
Pan, Peto, Henao-Restrepo, Repurposed antiviral drugs for COVID-19: interim WHO solidarity trial results, N Engl J Med
Rochwerg, Agarwal, Zeng, Remdesivir for severe COVID-19: a clinical practice guideline, BMJ
Spinner, Gottlieb, Criner, GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA
Verma, Hora, Jung, Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area, CMAJ
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Wunsch, Angus, Harrison, Variation in critical care services across North America and Western Europe, Crit Care Med
Yehya, Harhay, Curley, Reappraisal of ventilator-free days in critical care research, Am J Respir Crit Care Med
Yusuf, Wittes, Interpreting geographic variations in results of randomized, controlled trials, N Engl J Med
DOI record:
{
"DOI": "10.1503/cmaj.211698",
"ISSN": [
"0820-3946",
"1488-2329"
],
"URL": "http://dx.doi.org/10.1503/cmaj.211698",
"alternative-id": [
"10.1503/cmaj.211698"
],
"author": [
{
"affiliation": [],
"family": "Ali",
"given": "Karim",
"sequence": "first"
},
{
"affiliation": [],
"family": "Azher",
"given": "Tanweer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baqi",
"given": "Mahin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binnie",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borgia",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrier",
"given": "François M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cavayas",
"given": "Yiorgos Alexandroa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chagnon",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Matthew P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conly",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costiniuk",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daley",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Downey",
"given": "Catarina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duan",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duceppe",
"given": "Emmanuelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durand",
"given": "Madeleine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "English",
"given": "Shane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farjou",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fera",
"given": "Evradiki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fontela",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fowler",
"given": "Rob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fralick",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geagea",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grant",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harrison",
"given": "Luke B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Havey",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoang",
"given": "Holly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kelly",
"given": "Lauren E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keynan",
"given": "Yoav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khwaja",
"given": "Kosar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klein",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klein",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolan",
"given": "Christophe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kronfli",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamontagne",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lau",
"given": "Rob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fralick",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Todd C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Nelson",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Longo",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lostun",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacIntyre",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malhamé",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mangof",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGuinty",
"given": "Marlee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mergler",
"given": "Sonya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Munan",
"given": "Matthew P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murthy",
"given": "Srinivas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Neil",
"given": "Conar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ovakim",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papenburg",
"given": "Jesse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parhar",
"given": "Ken",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parvathy",
"given": "Seema Nair",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Chandni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez-Patrigeon",
"given": "Santiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinto",
"given": "Ruxandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajakumaran",
"given": "Subitha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rishu",
"given": "Asgar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roba-Oshin",
"given": "Malaika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rushton",
"given": "Moira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saleem",
"given": "Mariam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvadori",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scherr",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwartz",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Semret",
"given": "Makeda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silverman",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Ameeta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sligl",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Somayaji",
"given": "Ranjani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Darrell H.S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tobin",
"given": "Siobhan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Todd",
"given": "Meaghan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "Tuong-Vi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsang",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turgeon",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vakil",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weatherald",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yansouni",
"given": "Cedric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zarychanski",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Canadian Treatments for COVID-19 (CATCO)",
"sequence": "additional"
}
],
"container-title": "Canadian Medical Association Journal",
"container-title-short": "CMAJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
19
]
],
"date-time": "2022-01-19T14:15:19Z",
"timestamp": 1642601719000
},
"deposited": {
"date-parts": [
[
2022,
3,
4
]
],
"date-time": "2022-03-04T17:21:10Z",
"timestamp": 1646414470000
},
"indexed": {
"date-parts": [
[
2022,
10,
20
]
],
"date-time": "2022-10-20T05:22:08Z",
"timestamp": 1666243328484
},
"is-referenced-by-count": 22,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
1,
19
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2022,
2,
22
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
22
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1503/cmaj.211698",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "382",
"original-title": [],
"page": "E242-E251",
"prefix": "10.1503",
"published": {
"date-parts": [
[
2022,
1,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
22
]
]
},
"publisher": "CMA Impact Inc.",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.cmaj.ca/lookup/doi/10.1503/cmaj.211698"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial",
"type": "journal-article",
"volume": "194"
}