Aerosolized dornase alfa (DNase I) for the treatment of severe respiratory failure in COVID-19: a randomized controlled trial
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf246, NCT04541979, Apr 2025
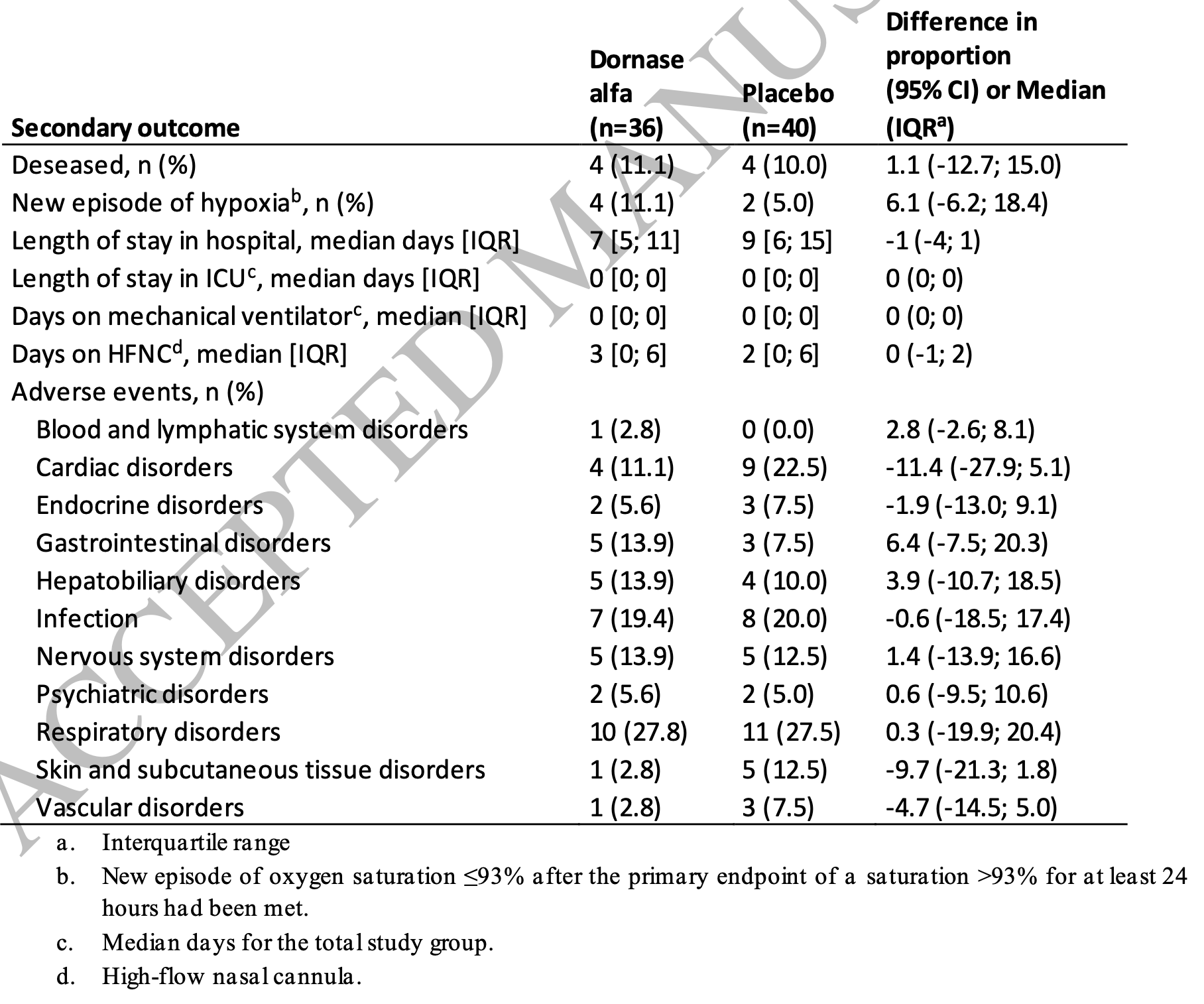
RCT 76 hospitalized COVID-19 patients showing no significant difference with inhaled dornase alfa (DNase I) for resolution of hypoxia or other clinical outcomes.
|
risk of death, 11.1% higher, RR 1.11, p = 1.00, treatment 4 of 36 (11.1%), control 4 of 40 (10.0%).
|
|
risk of no recovery, 2.0% higher, HR 1.02, p = 0.94, treatment 36, control 40, inverted to make HR<1 favor treatment, resolution of hypoxia, Kaplan-Meier, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Åkesson et al., 24 Apr 2025, Single Blind Randomized Controlled Trial, placebo-controlled, Sweden, peer-reviewed, mean age 60.5, 11 authors, study period 4 June, 2020 - 11 January, 2022, trial NCT04541979 (history).
Contact: adam.linder@med.lu.se.
Aerosolized dornase alfa (DNase I) for the treatment of severe respiratory failure in COVID-19: a randomized controlled trial
Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf246
Background: The lung injury in COVID-19 is characterized by neutrophil invasion and the release of neutrophil extracellular traps (NETs). An aberrant NET formation may induce local inflammation and increase sputum viscosity. Inhalation of DNase I (dornase alfa) is a treatment option that degrades NETs in the airways. Previous case series have indicated positive clinical effects of inhaled dornase alfa.
Methods: Patients admitted to the hospital with acute COVID-19 and hypoxia (oxygen saturation <90%) were randomly assigned to receive aerosolized dornase alfa twice daily for five days or a placebo in addition to standard-of-care. The primary outcome was discharge from the hospital or an oxygen saturation of >93% without respiratory support.
Conflict of interests: None
Author contributions PÅ helped initiate the study, enrolled patients and wrote the manuscript LM helped initiate the study, enrolled patients and critically revised the manuscript MR wrote the application for ethical permit and critically revised the manuscript MI helped iniate the study, enrolled patients and critically revised the manuscript SJ made the statistical analysis FM included patients in Malmö and critically revised the manuscript
References
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps, J Exp Med, doi:10.1084/jem.20200652
Barnes, Burnett, Blumenstein, Clark, Cuker et al., Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum, J Thromb Thrombolysis, doi:10.1007/s11239-020-02138-z
Bendib, De Chaisemartin, Mekontso Dessap, Chollet-Martin, De Prost, Understanding the Role of Neutrophil Extracellular Traps in Patients With Severe Pneumonia and ARDS, Chest, doi:10.1016/j.chest.2019.08.2179
Camporota, Cronin, Busana, Gattinoni, Formenti, Pathophysiology of coronavirus-19 disease acute lung injury, Curr Opin Crit Care, doi:10.1097/mcc.0000000000000911
Ebrahimi, Giaglis, Hahn, Blum, Baumgartner et al., Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial, Eur Respir J, doi:10.1183/13993003.01389-2017
Fisher, Mohanty, Karlsson, Khademi, Malmström et al., Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients, Mol Cell Proteomics, doi:10.1016/j.mcpro.2021.100113
Gavriilidis, Antoniadou, Chrysanthopoulou, Ntinopoulou, Smyrlis et al., Combined administration of inhaled DNase, baricitinib and tocilizumab as rescue treatment in COVID -19 patients with severe respiratory failure, Clin Immunol, doi:10.1016/j.clim.2022.109016
Gygi, Maguire, Patel, Shinde, Konstorum et al., Integrated longitudinal multiomics study identifies immune programs associated with acute COVID -19 severity and mortality, J Clin Invest, doi:10.1172/jci176640
Holliday, Earhart, Alnijoumi, Krvavac, Allen et al., Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to Covid-19, Front Immunol, doi:10.3389/fimmu.2021.714833
Jiménez-Alcázar, Rangaswamy, Panda, Bitterling, Simsek et al., Host DNases prevent vascular occlusion by neutrophil extracellular traps, Science, doi:10.1126/science.aam8897
Lefrançais, Mallavia, Zhuo, Calfee, Looney, Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury, JCI Insight, doi:10.1172/jci.insight.98178
Middleton, He, Denorme, Campbell, Ng et al., Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome, Blood, doi:10.1182/blood.2020007008
Mohanty, Fisher, Bakochi, Neumann, Cardoso et al., Neutrophil extracellular traps in the central nervous system hinder bacterial clearance during pneumococcal meningitis, Nat Commun, doi:10.1038/s41467-019-09040-0
Reyes, Murthy, Garcia-Gallo, Irvine, Merson et al., Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study, ERJ Open Res, doi:10.1183/23120541.00552-2021
Saffarzadeh, Juenemann, Queisser, Lochnit, Barreto et al., Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones, PLoS One, doi:10.1371/journal.pone.0032366
Scozzi, Liao, Krupnick, Kreisel, Gelman, The role of neutrophil extracellular traps in acute lung injury, Front Immunol, doi:10.3389/fimmu.2022.953195
Toma, Darwish, Taylor, Harlacher, Darwish, The Use of Dornase Alfa in the Management of COVID-19-Associated Adult Respiratory Distress Syndrome, Crit Care Res Pract, doi:10.1155/2021/8881115
Toussaint, Jackson, Swieboda, Guedán, Tsourouktsoglou et al., Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation, Nat Med, doi:10.1038/nm.4332
Weber, Chau, Egeblad, Barnes, Janowitz, Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID -19 patients: a case series, Mol Med, doi:10.1186/s10020-020-00215-w
DOI record:
{
"DOI": "10.1093/ofid/ofaf246",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf246",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The lung injury in COVID-19 is characterized by neutrophil invasion and the release of neutrophil extracellular traps (NETs). An aberrant NET formation may induce local inflammation and increase sputum viscosity. Inhalation of DNase I (dornase alfa) is a treatment option that degrades NETs in the airways. Previous case series have indicated positive clinical effects of inhaled dornase alfa.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Patients admitted to the hospital with acute COVID-19 and hypoxia (oxygen saturation &lt;90%) were randomly assigned to receive aerosolized dornase alfa twice daily for five days or a placebo in addition to standard-of-care. The primary outcome was discharge from the hospital or an oxygen saturation of &gt;93% without respiratory support.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 76 patients were randomized. The study was stopped when the omicron virus variant appeared. The clinical response rate did not differ between patients receiving the active substance or placebo. Secondary outcomes such as mortality, a new episode of hypoxia, length of stay in the hospital, and adverse events were similar across groups. A subanalysis of patients older or younger than 65 showed no differences in primary or secondary outcomes.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Aerosolized dornase alfa failed to improve hypoxia in hospitalized patients with acute COVID-19. The study was conducted during a time of heterogeneity in viral variants and vaccination status of participants. Whether dornase alfa affects the outcomes in other respiratory infections requires further study.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Lund University, Department of Clinical Sciences Lund, Division of Infection Medicine and Skåne University Hospital, Department of Infectious Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"family": "Åkesson",
"given": "Per",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-7623-0342",
"affiliation": [
{
"name": "Lund University, Department of Clinical Sciences Lund, Division of Infection Medicine and Skåne University Hospital, Department of Infectious Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Mellhammar",
"given": "Lisa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1588-5473",
"affiliation": [
{
"name": "Lund University, Department of Clinical Sciences Lund, Division of Infection Medicine and Skåne University Hospital, Department of Infectious Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Rasmussen",
"given": "Magnus",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2995-1312",
"affiliation": [
{
"name": "Lund University, Department of Clinical Sciences Lund, Division of Infection Medicine and Skåne University Hospital, Department of Infectious Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Inghammar",
"given": "Malin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Skåne University Hospital, Clinical Studies Sweden - Forum South , Lund ,",
"place": [
"Sweden"
]
}
],
"family": "Jesperson",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Skåne University Hospital, Department of Infectious Diseases , Malmö ,",
"place": [
"Sweden"
]
}
],
"family": "Månsson",
"given": "Fredrik",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0006-6296-2522",
"affiliation": [
{
"name": "Central Hospital of Kristianstad, Department of Infectious Diseases. Kristianstad ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Economou Lundeberg",
"given": "Elin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Central Hospital of Kristianstad, Department of Infectious Diseases. Kristianstad ,",
"place": [
"Sweden"
]
},
{
"name": "Clinical Infection Medicine, Dept of Translational Medicine, Lund University , Malmö ,",
"place": [
"Sweden"
]
}
],
"family": "Walles",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Skåne University Hospital, Department of Pulmonary Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"family": "Wallberg",
"given": "Martin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0155-4828",
"affiliation": [
{
"name": "Skåne University Hospital, Department of Anesthesiology and Intensive Care , Lund ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Frigyesi",
"given": "Attila",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8187-7239",
"affiliation": [
{
"name": "Lund University, Department of Clinical Sciences Lund, Division of Infection Medicine and Skåne University Hospital, Department of Infectious Diseases , Lund ,",
"place": [
"Sweden"
]
}
],
"authenticated-orcid": false,
"family": "Linder",
"given": "Adam",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
4,
24
]
],
"date-time": "2025-04-24T11:49:28Z",
"timestamp": 1745495368000
},
"deposited": {
"date-parts": [
[
2025,
4,
24
]
],
"date-time": "2025-04-24T11:49:29Z",
"timestamp": 1745495369000
},
"indexed": {
"date-parts": [
[
2025,
4,
24
]
],
"date-time": "2025-04-24T12:10:07Z",
"timestamp": 1745496607140,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
4,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
24
]
],
"date-time": "2025-04-24T00:00:00Z",
"timestamp": 1745452800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf246/62997559/ofaf246.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf246/62997559/ofaf246.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
4,
24
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
24
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofaf246/8119012"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Aerosolized dornase alfa (DNase I) for the treatment of severe respiratory failure in COVID-19: a randomized controlled trial",
"type": "journal-article"
}