Combined administration of inhaled DNase, baricitinib and tocilizumab as rescue treatment in COVID-19 patients with severe respiratory failure
et al., Clinical Immunology, doi:10.1016/j.clim.2022.109016, NCT05279391, Apr 2022
Budesonide for COVID-19
28th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small retrospective study of hospitalized patients with severe respiratory failure, 22 treated with a combination of budesonide, tocilizumab, baricitinib, dornase alfa, and salbutamol or/and ipratropium, and 26 SOC patients, showing lower mortality and intubation with treatment. The average age of the combined treatment group was 6 years younger than the SOC group.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
This study is excluded in meta-analysis:
combined treatments may contribute more to the effect seen.
Study covers budesonide and dornase alfa.
|
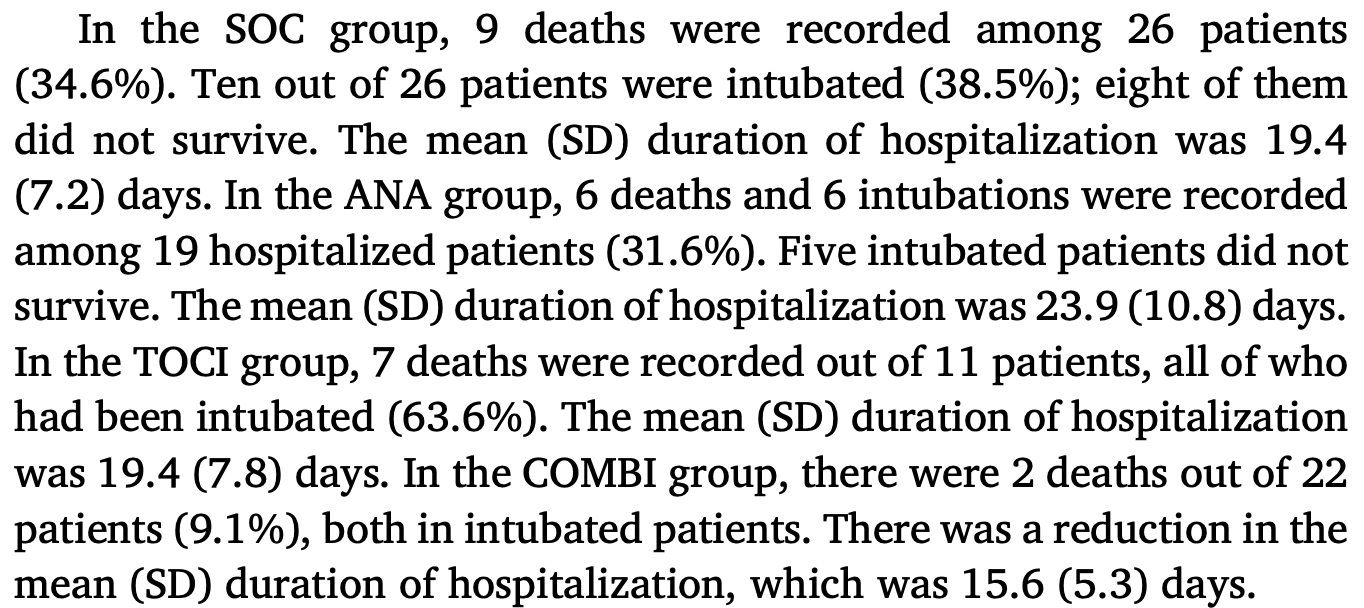
risk of death, 73.7% lower, RR 0.26, p = 0.045, treatment 2 of 22 (9.1%), control 9 of 26 (34.6%), NNT 3.9.
|
|
risk of mechanical ventilation, 76.4% lower, RR 0.24, p = 0.02, treatment 2 of 22 (9.1%), control 10 of 26 (38.5%), NNT 3.4.
|
|
hospitalization time, 19.6% lower, relative time 0.80, p = 0.02, treatment mean 15.6 (±5.3) n=22, control mean 19.4 (±7.2) n=26.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gavriilidis et al., 18 Apr 2022, retrospective, Greece, peer-reviewed, 19 authors, study period 25 October, 2020 - 18 August, 2021, average treatment delay 10.6 days, this trial uses multiple treatments in the treatment arm (combined with tocilizumab, baricitinib, dornase alfa, and salbutamol or/and ipratropium) - results of individual treatments may vary, trial NCT05279391 (history).
Contact: pskendro@med.duth.gr, kritis@med.duth.gr.
Combined administration of inhaled DNase, baricitinib and tocilizumab as rescue treatment in COVID-19 patients with severe respiratory failure
Clinical Immunology, doi:10.1016/j.clim.2022.109016
Aiming to reduce mortality in COVID-19 with severe respiratory failure we administered a combined rescue treatment (COMBI) on top of standard-of-care (SOC: dexamethasone/heparin) consisted of inhaled DNase to dissolve thrombogenic neutrophil extracellular traps, plus agents against cytokine-mediated hyperinflammation, namely anti-IL-6-receptor tocilizumab and JAK1/2 inhibitor baricitinib. Patients with PaO2/FiO2 < 100 mmHg were analysed. COMBI group (n = 22) was compared with similar groups that had received SOC alone (n = 26) or SOC plus monotherapy with either IL-1-receptor antagonist anakinra (n = 19) or tocilizumab (n = 11). COMBI was significantly associated with lower in-hospital mortality and intubation rate, shorter duration of hospitalization, and prolonged overall survival after a median follow-up of 110 days. In vitro, COVID-19 plasma induced tissue factor/thrombin pathway in primary lung fibroblasts. This effect was inhibited by the immunomodulatory agents of COMBI providing a mechanistic explanation for the clinical observations. These results support the conduct of randomized trials using combined immunomodulation in COVID-19 to target multiple interconnected pathways of immunothrombosis.
Declaration of Competing Interest None.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.clim.2022.109016.
References
Ackermann, Anders, Bilyy, Bowlin, Daniel et al., Patients with COVID-19: in the dark-NETs of neutrophils, Cell Death Differ, doi:10.1038/s41418-021-00805-z
Berlin, Gulick, Martinez, Severe Covid-19, N. Engl. J. Med, doi:10.1056/NEJMcp2009575
Bonaventura, Vecchié, Dagna, Martinod, Dixon et al., Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-021-00536-9
Borczuk, Salvatore, Seshan, Patel, Bussel et al., COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and new York City, Mod. Pathol, doi:10.1038/s41379-020-00661-1
Cate, Surviving Covid-19 with heparin?, N. Engl. J. Med, doi:10.1056/NEJMe2111151
Chrysanthopoulou, Gkaliagkousi, Lazaridis, Arelaki, Pateinakis et al., Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension, JCI Insight, doi:10.1172/jci.insight.148668
Chrysanthopoulou, Mitroulis, Kambas, Skendros, Kourtzelis et al., Tissue factor-thrombin signaling enhances the fibrotic activity of myofibroblasts in systemic sclerosis through up-regulation of endothelin receptor A, Arthritis Rheum, doi:10.1002/art.30586
Cruz, Mendes-Frias, Oliveira, Dias, Matos et al., Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia, Front. Immunol, doi:10.3389/fimmu.2021.613422
Cugno, Meroni, Gualtierotti, Griffini, Grovetti et al., Complement activation in patients with COVID-19: a novel therapeutic target, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2020.05.006
Delorey, Ziegler, Heimberg, Normand, Yang et al., COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets, Nature, doi:10.1038/s41586-021-03570-8
Freda, Yin, Ghebrehiwet, Rubenstein, SARS-CoV-2 proteins regulate inflammatory, thrombotic and diabetic responses in human arterial fibroblasts, Clin. Immunol, doi:10.1016/j.clim.2021.108733
Fuchs, Brill, Duerschmied, Schatzberg, Monestier et al., Extracellular DNA traps promote thrombosis, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.1005743107
Grant, Estrada, Ayala-Marin, Alvidrez-Camacho, Rodriguez et al., The many faces of JAKs and STATs within the COVID-19 storm, Front. Immunol, doi:10.3389/fimmu.2021.690477
Gustine, Jones, Immunopathology of hyperinflammation in COVID-19, Am. J. Pathol, doi:10.1016/j.ajpath.2020.08.009
Holliday, Earhart, Alnijoumi, Krvavac, Allen et al., Non-randomized trial of Dornase alfa for acute respiratory distress syndrome secondary to Covid-19, Front. Immunol, doi:10.3389/fimmu.2021.714833
Horby, Lim, Emberson, Mafham, Bell et al., RECOVERY Collaborative Group, Dexamethasone in hospitalized patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Howell, Goldsack, Marshall, Mcanulty, Starke et al., Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycininduced pulmonary fibrosis, Am. J. Pathol, doi:10.1016/S0002-9440(10)62525-4
Huet, Beaussier, Voisin, Jouveshomme, Dauriat et al., Anakinra for severe forms of COVID-19: a cohort study, Rheumatol, doi:10.1016/S2665-9913(20)30164-8
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 receptor antagonists in critically ill patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2100433
Kaiser, Leunig, Pekayvaz, Popp, Joppich et al., Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19, JCI Insight, doi:10.1172/jci.insight.150862
Kaklamanos, Belogiannis, Skendros, Gorgoulis, Vlachoyiannopoulos et al., COVID-19 Immunobiology: lessons learned, New Questions Arise, Frontiers in Immunology, doi:10.3389/fimmu.2021.719023
Kambas, Chrysanthopoulou, Vassilopoulos, Apostolidou, Skendros et al., Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2013-203430
Kircheis, Haasbach, Lueftenegger, Heyken, Ocker et al., NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients, Front. Immunol, doi:10.3389/fimmu.2020.598444
Leleu, Levionnois, Laurent, Lazaro, Richez et al., Elevated circulatory levels of microparticles are associated to lung fibrosis and vasculopathy during systemic sclerosis, Front. Immunol, doi:10.3389/fimmu.2020.532177
Linklater, Lawton, Fielding, Macaulay, Carroll et al., Introducing the palliative performance scale to clinicians: the Grampian experience, BMJ Support. Palliat. Care, doi:10.1136/bmjspcare-2011-000133
Lu, Liu, Chen, Yang, Hu et al., Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment, Signal Transduct Target Ther, doi:10.1038/s41392-021-00517-3
Luo, Li, Jiang, Chen, Wang et al., Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19, Trends Pharmacol. Sci, doi:10.1016/j.tips.2020.06.007
Malik, Patel, Mehta, Patel, Kelkar et al., Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis, BMJ Evid Based Med, doi:10.1136/bmjebm-2020-111536
Marconi, Ramanan, Bono, Kartman, Krishnan et al., Study Group, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00331-3
Mcfadyen, Stevens, Peter, The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications, Circ. Res, doi:10.1161/CIRCRESAHA.120.317447
Melms, Biermann, Huang, Wang, Nair et al., A molecular single-cell lung atlas of lethal COVID-19, Nature, doi:10.1038/s41586-021-03569-1
Mitroulis, Kambas, Anyfanti, Doumas, Ritis, The multivalent activity of the tissue factor-thrombin pathway in thrombotic and non-thrombotic disorders as a target for therapeutic intervention, Expert Opin. Ther. Targets, doi:10.1517/14728222.2011.532788
Mitsios, Arampatzioglou, Arelaki, Mitroulis, Ritis, NETopathies? Unraveling the dark side of old diseases through neutrophils, Front Immunol, doi:10.3389/fimmu.2016.00678
Montero, Milara, Roger, Cortijo, Role of JAK/STAT in interstitial lung diseases, Mol. Cellular Mech. Int J Mol Sci, doi:10.3390/ijms22126211
Ren, Tong, Qiu, Ye, Wu et al., MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression, J Extracell Vesicles, doi:10.1080/20013078.2019.1698795
Rendeiro, Ravichandran, Bram, Chandar, Kim et al., The spatial landscape of lung pathology during COVID-19 progression, Nature, doi:10.1038/s41586-021-03475-6
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., And the Northwell COVID-19 research consortium, presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area, JAMA, doi:10.1001/jama.2020.6775
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in patients hospitalized with Covid-19 pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2030340
Satarker, Tom, Shaji, Alosious, Luvis et al., JAK-STAT pathway inhibition and their implications in COVID-19 therapy, Postgrad. Med, doi:10.1080/00325481.2020.1855921
Schoergenhofer, Schwameis, Wohlfarth, Brostjan, Abrams et al., Granulocyte colony-stimulating factor (G-CSF) increases histonecomplexed DNA plasma levels in healthy volunteers, Clin. Exp. Med, doi:10.1007/s10238-016-0413-6
Sharma, Inferring molecular mechanisms of dexamethasone therapy in severe COVID-19 from existing transcriptomic data, Gene, doi:10.1016/j.gene.2021.145665
Sinha, Rosin, Arora, Labit, Jaffer et al., Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19, Nat. Med, doi:10.1038/s41591-021-01576-3
Skendros, Mitsios, Chrysanthopoulou, Mastellos, Metallidis et al., Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis, J. Clin. Invest, doi:10.1172/JCI141374
Stebbing, Phelan, Griffin, Tucker, Oechsle et al., COVID-19: combining antiviral and anti-inflammatory treatments, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30132-8
Su, Wang, Yoo, Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2, Sci. Rep, doi:10.1038/s41598-021-92941-2
Synolaki, Papadopoulos, Divolis, Tsahouridou, Gavriilidis et al., The activin/follistatin axis is severely deregulated in COVID-19 and independently associated with in-hospital mortality, J. Infect. Dis, doi:10.1093/infdis/jiab108
Tanaka, Narazaki, Kishimoto, IL-6 in inflammation, immunity, and disease, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a016295
Toma, Darwish, Taylor, Harlacher, Darwish, The use of Dornase alfa in the management of COVID-19-associated adult respiratory distress syndrome, Crit. Care Res. Pract, doi:10.1155/2021/8881115
Valdebenito, Bessis, Annane, Lorin De La Grandmaison, Cramer-Bordé et al., COVID-19 lung pathogenesis in SARS-CoV-2 autopsy cases, Front. Immunol, doi:10.3389/fimmu.2021.735922
Wu, Mcgoogan, Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Yan, Freiwald, Chauss, Wang, West et al., SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation, Sci. Immunol, doi:10.1126/sciimmunol.abg0833
Yin, Yu, Xie, Dai, Dong et al., Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein, Cell. Signal, doi:10.1016/j.cellsig.2021.110029
Yu, He, Wu, Xie, Liu et al., Dysregulated adaptive immune response contributes to severe COVID-19, Cell Res, doi:10.1038/s41422-020-0391-9
Zhang, Zhang, Qiao, Zhang, Qi, Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.106749
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1016/j.clim.2022.109016",
"ISSN": [
"1521-6616"
],
"URL": "http://dx.doi.org/10.1016/j.clim.2022.109016",
"alternative-id": [
"S1521661622000973"
],
"article-number": "109016",
"author": [
{
"affiliation": [],
"family": "Gavriilidis",
"given": "Efstratios",
"sequence": "first"
},
{
"affiliation": [],
"family": "Antoniadou",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chrysanthopoulou",
"given": "Akrivi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ntinopoulou",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smyrlis",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fotiadou",
"given": "Iliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zioga",
"given": "Nikoleta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kogias",
"given": "Dionysios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Natsi",
"given": "Anastasia-Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelekoudas",
"given": "Christos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Satiridou",
"given": "Evangelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bakola",
"given": "Stefania-Aspasia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papagoras",
"given": "Charalampos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mitroulis",
"given": "Ioannis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peichamperis",
"given": "Paschalis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mikroulis",
"given": "Dimitrios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papadopoulos",
"given": "Vasileios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Skendros",
"given": "Panagiotis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ritis",
"given": "Konstantinos",
"sequence": "additional"
}
],
"container-title": "Clinical Immunology",
"container-title-short": "Clinical Immunology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
18
]
],
"date-time": "2022-04-18T15:48:39Z",
"timestamp": 1650296919000
},
"deposited": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T17:40:46Z",
"timestamp": 1650562846000
},
"indexed": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T21:42:19Z",
"timestamp": 1650577339297
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1521661622000973?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1521661622000973?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "109016",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA.",
"key": "10.1016/j.clim.2022.109016_bb0005",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009575",
"article-title": "Severe Covid-19",
"author": "Berlin",
"doi-asserted-by": "crossref",
"first-page": "2451",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.clim.2022.109016_bb0010",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/j.clim.2022.109016_bb0015",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "And the Northwell COVID-19 research consortium, presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA.",
"key": "10.1016/j.clim.2022.109016_bb0020",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.clim.2022.109016_bb0025",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMe2111151",
"article-title": "Surviving Covid-19 with heparin?",
"author": "ten Cate",
"doi-asserted-by": "crossref",
"first-page": "845",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.clim.2022.109016_bb0030",
"volume": "385",
"year": "2021"
},
{
"article-title": "COVID-19 Immunobiology: lessons learned",
"author": "Kaklamanos",
"first-page": "3431",
"journal-title": "New Questions Arise, Frontiers in Immunology.",
"key": "10.1016/j.clim.2022.109016_bb0035",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03475-6",
"article-title": "The spatial landscape of lung pathology during COVID-19 progression",
"author": "Rendeiro",
"doi-asserted-by": "crossref",
"first-page": "564",
"journal-title": "Nature.",
"key": "10.1016/j.clim.2022.109016_bb0040",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03570-8",
"article-title": "COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets",
"author": "Delorey",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Nature.",
"key": "10.1016/j.clim.2022.109016_bb0045",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1016/j.ajpath.2020.08.009",
"article-title": "Immunopathology of hyperinflammation in COVID-19",
"author": "Gustine",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Am. J. Pathol.",
"key": "10.1016/j.clim.2022.109016_bb0050",
"volume": "191",
"year": "2021"
},
{
"DOI": "10.1172/JCI141374",
"article-title": "Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis",
"author": "Skendros",
"doi-asserted-by": "crossref",
"first-page": "6151",
"journal-title": "J. Clin. Invest.",
"key": "10.1016/j.clim.2022.109016_bb0055",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.690477",
"article-title": "The many faces of JAKs and STATs within the COVID-19 storm",
"author": "Grant",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0060",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.05.006",
"article-title": "Complement activation in patients with COVID-19: a novel therapeutic target",
"author": "Cugno",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0065",
"volume": "146",
"year": "2020"
},
{
"article-title": "Anakinra for severe forms of COVID-19: a cohort study",
"author": "Huet",
"first-page": "e393",
"journal-title": "Rheumatol.",
"key": "10.1016/j.clim.2022.109016_bb0070",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with Covid-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.clim.2022.109016_bb0075",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2020.106749",
"article-title": "Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.clim.2022.109016_bb0080",
"volume": "86",
"year": "2020"
},
{
"article-title": "The use of Dornase alfa in the management of COVID-19-associated adult respiratory distress syndrome",
"author": "Toma",
"journal-title": "Crit. Care Res. Pract.",
"key": "10.1016/j.clim.2022.109016_bb0085",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.714833",
"article-title": "Non-randomized trial of Dornase alfa for acute respiratory distress syndrome secondary to Covid-19",
"author": "Holliday",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0090",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/bmjspcare-2011-000133",
"article-title": "Introducing the palliative performance scale to clinicians: the Grampian experience",
"author": "Linklater",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "BMJ Support. Palliat. Care",
"key": "10.1016/j.clim.2022.109016_bb0095",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.1038/s41392-021-00517-3",
"article-title": "Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "106",
"journal-title": "Signal Transduct Target Ther.",
"key": "10.1016/j.clim.2022.109016_bb0100",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab108",
"article-title": "The activin/follistatin axis is severely deregulated in COVID-19 and independently associated with in-hospital mortality",
"author": "Synolaki",
"doi-asserted-by": "crossref",
"first-page": "1544",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.clim.2022.109016_bb0105",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1136/bmjebm-2020-111536",
"article-title": "Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis",
"author": "Malik",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "BMJ Evid Based Med.",
"key": "10.1016/j.clim.2022.109016_bb0110",
"volume": "26",
"year": "2021"
},
{
"article-title": "Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19",
"author": "Bonaventura",
"first-page": "1",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0115",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.532177",
"article-title": "Elevated circulatory levels of microparticles are associated to lung fibrosis and vasculopathy during systemic sclerosis",
"author": "Leleu",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0120",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/art.30586",
"article-title": "Tissue factor-thrombin signaling enhances the fibrotic activity of myofibroblasts in systemic sclerosis through up-regulation of endothelin receptor A",
"author": "Chrysanthopoulou",
"doi-asserted-by": "crossref",
"first-page": "3586",
"journal-title": "Arthritis Rheum.",
"key": "10.1016/j.clim.2022.109016_bb0125",
"volume": "63",
"year": "2011"
},
{
"DOI": "10.1016/S0002-9440(10)62525-4",
"article-title": "Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis",
"author": "Howell",
"doi-asserted-by": "crossref",
"first-page": "1383",
"journal-title": "Am. J. Pathol.",
"key": "10.1016/j.clim.2022.109016_bb0130",
"volume": "159",
"year": "2001"
},
{
"DOI": "10.1517/14728222.2011.532788",
"article-title": "The multivalent activity of the tissue factor-thrombin pathway in thrombotic and non-thrombotic disorders as a target for therapeutic intervention",
"author": "Mitroulis",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Expert Opin. Ther. Targets",
"key": "10.1016/j.clim.2022.109016_bb0135",
"volume": "15",
"year": "2011"
},
{
"DOI": "10.3389/fimmu.2020.598444",
"article-title": "NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients",
"author": "Kircheis",
"doi-asserted-by": "crossref",
"first-page": "3446",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0140",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.613422",
"article-title": "Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia",
"author": "Santa Cruz",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0145",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-92941-2",
"article-title": "Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "13464",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.clim.2022.109016_bb0150",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.abg0833",
"article-title": "SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "eabg0833",
"journal-title": "Sci. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0155",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1007/s10238-016-0413-6",
"article-title": "Granulocyte colony-stimulating factor (G-CSF) increases histone-complexed DNA plasma levels in healthy volunteers",
"author": "Schoergenhofer",
"doi-asserted-by": "crossref",
"first-page": "243",
"journal-title": "Clin. Exp. Med.",
"key": "10.1016/j.clim.2022.109016_bb0160",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1172/jci.insight.150862",
"article-title": "Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19",
"author": "Kaiser",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight.",
"key": "10.1016/j.clim.2022.109016_bb0165",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41418-021-00805-z",
"article-title": "Patients with COVID-19: in the dark-NETs of neutrophils",
"author": "Ackermann",
"doi-asserted-by": "crossref",
"first-page": "3125",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.clim.2022.109016_bb0170",
"volume": "28",
"year": "2021"
},
{
"article-title": "NETopathies? Unraveling the dark side of old diseases through neutrophils",
"author": "Mitsios",
"first-page": "678",
"journal-title": "Front Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0175",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1101/cshperspect.a016295",
"article-title": "IL-6 in inflammation, immunity, and disease",
"author": "Tanaka",
"doi-asserted-by": "crossref",
"journal-title": "Cold Spring Harb. Perspect. Biol.",
"key": "10.1016/j.clim.2022.109016_bb0180",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.3390/ijms22126211",
"article-title": "Role of JAK/STAT in interstitial lung diseases",
"author": "Montero",
"doi-asserted-by": "crossref",
"first-page": "6211",
"journal-title": "Mol. Cellular Mech. Int J Mol Sci.",
"key": "10.1016/j.clim.2022.109016_bb0185",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41379-020-00661-1",
"article-title": "COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and new York City",
"author": "Borczuk",
"doi-asserted-by": "crossref",
"first-page": "2156",
"journal-title": "Mod. Pathol.",
"key": "10.1016/j.clim.2022.109016_bb0190",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317447",
"article-title": "The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications",
"author": "McFadyen",
"doi-asserted-by": "crossref",
"first-page": "571",
"journal-title": "Circ. Res.",
"key": "10.1016/j.clim.2022.109016_bb0195",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1016/j.gene.2021.145665",
"article-title": "Inferring molecular mechanisms of dexamethasone therapy in severe COVID-19 from existing transcriptomic data",
"author": "Sharma",
"doi-asserted-by": "crossref",
"journal-title": "Gene.",
"key": "10.1016/j.clim.2022.109016_bb0200",
"volume": "788",
"year": "2021"
},
{
"article-title": "Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19",
"author": "Sinha",
"journal-title": "Nat. Med.",
"key": "10.1016/j.clim.2022.109016_bb0205",
"year": "2021"
},
{
"article-title": "Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial, lancet",
"first-page": "295",
"journal-title": "Respir. Med.",
"key": "10.1016/j.clim.2022.109016_bb0210",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19",
"author": "REMAP-CAP Investigators",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.clim.2022.109016_bb0215",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet.",
"key": "10.1016/j.clim.2022.109016_bb0220",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1073/pnas.1005743107",
"article-title": "Extracellular DNA traps promote thrombosis",
"author": "Fuchs",
"doi-asserted-by": "crossref",
"first-page": "15880",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.clim.2022.109016_bb0225",
"volume": "107",
"year": "2010"
},
{
"DOI": "10.1016/j.tips.2020.06.007",
"article-title": "Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Trends Pharmacol. Sci.",
"key": "10.1016/j.clim.2022.109016_bb0230",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1080/00325481.2020.1855921",
"article-title": "JAK-STAT pathway inhibition and their implications in COVID-19 therapy",
"author": "Satarker",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "Postgrad. Med.",
"key": "10.1016/j.clim.2022.109016_bb0235",
"volume": "133",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.clim.2022.109016_bb0240",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"article-title": "COVID-19: combining antiviral and anti-inflammatory treatments",
"author": "Stebbing",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.clim.2022.109016_bb0245",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0391-9",
"article-title": "Dysregulated adaptive immune response contributes to severe COVID-19",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "814",
"journal-title": "Cell Res.",
"key": "10.1016/j.clim.2022.109016_bb0250",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03569-1",
"article-title": "A molecular single-cell lung atlas of lethal COVID-19",
"author": "Melms",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Nature.",
"key": "10.1016/j.clim.2022.109016_bb0255",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.735922",
"article-title": "COVID-19 lung pathogenesis in SARS-CoV-2 autopsy cases",
"author": "Valdebenito",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0260",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2021.108733",
"article-title": "SARS-CoV-2 proteins regulate inflammatory, thrombotic and diabetic responses in human arterial fibroblasts",
"author": "Freda",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Immunol.",
"key": "10.1016/j.clim.2022.109016_bb0265",
"volume": "227",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.148668",
"article-title": "Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension",
"author": "Chrysanthopoulou",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight.",
"key": "10.1016/j.clim.2022.109016_bb0270",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.cellsig.2021.110029",
"article-title": "Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein",
"author": "Yin",
"doi-asserted-by": "crossref",
"journal-title": "Cell. Signal.",
"key": "10.1016/j.clim.2022.109016_bb0275",
"volume": "84",
"year": "2021"
},
{
"DOI": "10.1080/20013078.2019.1698795",
"article-title": "MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression",
"author": "Ren",
"doi-asserted-by": "crossref",
"first-page": "1698795",
"journal-title": "J Extracell Vesicles.",
"key": "10.1016/j.clim.2022.109016_bb0280",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2013-203430",
"article-title": "Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease",
"author": "Kambas",
"doi-asserted-by": "crossref",
"first-page": "1854",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.clim.2022.109016_bb0285",
"volume": "73",
"year": "2014"
}
],
"reference-count": 57,
"references-count": 57,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1521661622000973"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Combined administration of inhaled DNase, baricitinib and tocilizumab as rescue treatment in COVID-19 patients with severe respiratory failure",
"type": "journal-article",
"volume": "238"
}
