Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2023.1147980, Mar 2023
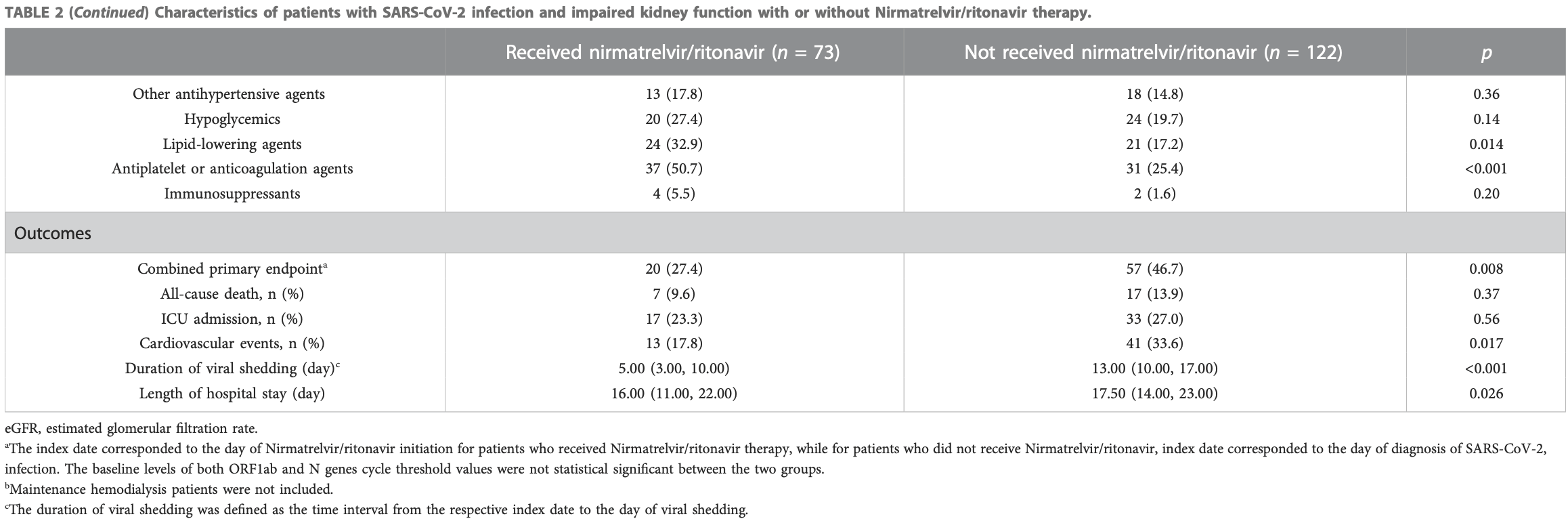
Retrospective 195 patients with impaired kidney function in China, showing lower combined mortality/ICU/cardiovascular events, and improved viral clearance with paxlovid.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
risk of death, 31.2% lower, RR 0.69, p = 0.50, treatment 7 of 73 (9.6%), control 17 of 122 (13.9%), NNT 23.
|
|
mortality/ICU/cardiovascular events, 44.0% lower, HR 0.56, p = 0.04, treatment 73, control 122, adjusted per study, multivariable, model 2, primary outcome.
|
|
risk of ICU admission, 13.9% lower, RR 0.86, p = 0.61, treatment 17 of 73 (23.3%), control 33 of 122 (27.0%), NNT 27.
|
|
hospitalization time, 8.6% lower, relative time 0.91, p = 0.03, treatment 73, control 122.
|
|
time to viral-, 61.5% lower, relative time 0.38, p = 0.001, treatment 73, control 122.
|
|
risk of no viral clearance, 73.0% lower, HR 0.27, p < 0.001, treatment 73, control 122, adjusted per study, inverted to make HR<1 favor treatment, viral shedding, multivariable, model 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Yan et al., 22 Mar 2023, retrospective, placebo-controlled, China, peer-reviewed, median age 74.0, 10 authors, study period 1 April, 2022 - 30 June, 2022.
Contact: shan_mou@shsmu.edu.cn, guleyi@aliyun.com, monsoon585@foxmail.com.
Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge
Frontiers in Pharmacology, doi:10.3389/fphar.2023.1147980
Background: Nirmatrelvir/ritonavir has demonstrated effectiveness in high-risk patients with coronavirus disease 2019 . However, investigations on the efficacy and safety of nirmatrelvir/ritonavir in patients with kidney dysfunction are limited. Methods: Data were collected from the patients admitted to a COVID-19 referral center in Shanghai, China. Patients were at least 18 years of age and had a baseline estimated glomerular filtration rate (eGFR) of <60 ml/min/1•73 m 2 . The primary endpoint was a composite of all-cause mortality, intensive care unit admission, or cardiovascular events. The secondary endpoint was viral shedding. Results: Among the 195 participants, 73 received nirmatrelvir/ritonavir. A lower risk of the primary endpoint was observed in nirmatrelvir/ritonavir recipients compared with non-recipients [adjusted HR 0.56 (95% CI: 0.32-0.96); p = 0.035]. Nirmatrelvir/ritonavir recipients experienced a shorter duration of viral shedding ; p < 0.001) and faster viral load clearance versus non-recipients. Among the nirmatrelvir/ritonavir users, earlier initiation of nirmatrelvir/ritonavir within 5 days since COVID-19 diagnosis was related with shorter viral shedding time ; p < 0.001) compared to late initiation. No patients reported serious adverse events during treatment.
Conclusion: Our findings support the early initiation of nirmatrelvir/ritonavir for high-risk patients with impaired kidney function. This could improve patient outcomes and shorten the viral shedding period.
Ethics statement The studies involving human participants were reviewed and approved by Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. The ethics committee waived the requirement of written informed consent for participation.
Author contributions JY, SM, and LG designed the study. JY, HC, and JW analyzed the data and revised the manuscript. JY and SM drafted and edited the final manuscript. All authors took part in collecting and verifying the
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1147980/ full#supplementary-material
References
Arbel, Wolff Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe covid-19 outcomes during the omicron surge, N. Engl. J. Med, doi:10.1056/NEJMoa2204919
Carlson, Nelveg-Kristensen, Freese Ballegaard, Feldt-Rasmussen, Hornum et al., Increased vulnerability to COVID-19 in chronic kidney disease, J. Intern Med, doi:10.1111/joim.13239
Christensen, Olsen, Long, Snehal, Davis et al., Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in houston, Texas, Am. J. Pathol, doi:10.1016/j.ajpath.2022.01.007
Dejnirattisai, Huo, Zhou, Zahradnik, Supasa et al., None
Gnanenthiran, Borghi, Burger, Caramelli, Charchar et al., Renin-angiotensin system inhibitors in patients with COVID-19: A metaanalysis of randomized controlled trials led by the international society of hypertension, J. Am. Heart Assoc, doi:10.1161/JAHA.122.026143
Graham, Daily briefing: Omicron coronavirus variant puts scientists on alert, Nature, doi:10.1038/d41586-021-03564-6
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET), Nat. Rev. Microbiol, doi:10.1093/cid/ciaa1012
Levey, Stevens, Schmid, Zhang, Castro et al., None
Lingscheid, Kinzig, Kruger, Muller, Bolke et al., Pharmacokinetics of nirmatrelvir and ritonavir in COVID-19 patients with endstage renal disease on intermittent hemodialysis, Antimicrob. Agents Chemother
Lu, Zhang, Zhang, Ai, He et al., Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave, Emerg. Microbes Infect, doi:10.1080/22221751.2022.2109517
Mahase, Nahhas, Webster, Owen, The promising use of nanomolecular imprinted templates for improved SARS-CoV-2 detection, drug delivery and research, doi:10.1126/science.abl4784
Ozturk, Turgutalp, Arici, Odabas, Altiparmak et al., Mortality analysis of COVID-19 infection in chronic kidney disease, Frontiers in Pharmacology frontiersin
Qiao, Li, Zeng, Liu, Luo et al., SARS-CoV
Russo, Esposito, Taramasso, Magnasco, Saio et al., Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy, J. Nephrol, doi:10.1007/s40620-020-00875-1
Toussi, Neutel, Navarro, Preston, Shi et al., Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID-19, in subjects with renal impairment, Clin. Pharmacol. Ther, doi:10.1002/cpt.2688
Wang, Peng, Ye, Li, Li et al., Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension, Front. Med, doi:10.1007/s11684-021-0850-9
Zhang, Wang, Ning, He, Wang, Protecting older people: A high priority during the COVID-19 pandemic, Lancet, doi:10.1016/S0140-6736(22)01530-6
Zhang, Zhang, Chen, Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic, Lancet, doi:10.1016/S0140-6736(22)00838-8
Zhang, Zhou ; Flythe, Assimon, Tugman, Chang et al., Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States, doi:10.1002/jmv.27491
Zhu, Xiao, Meng, Tang, Li et al., Nanovesicles derived from bispecific CAR-T cells targeting the spike protein of SARS-CoV-2 for treating COVID-19, J. Nanobiotechnology, doi:10.1186/s12951-021-01148-0
DOI record:
{
"DOI": "10.3389/fphar.2023.1147980",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2023.1147980",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> Nirmatrelvir/ritonavir has demonstrated effectiveness in high-risk patients with coronavirus disease 2019 (COVID-19). However, investigations on the efficacy and safety of nirmatrelvir/ritonavir in patients with kidney dysfunction are limited.</jats:p><jats:p><jats:bold>Methods:</jats:bold> Data were collected from the patients admitted to a COVID-19 referral center in Shanghai, China. Patients were at least 18 years of age and had a baseline estimated glomerular filtration rate (eGFR) of &lt;60 ml/min/1·73 m<jats:sup>2</jats:sup>. The primary endpoint was a composite of all-cause mortality, intensive care unit admission, or cardiovascular events. The secondary endpoint was viral shedding.</jats:p><jats:p><jats:bold>Results:</jats:bold> Among the 195 participants, 73 received nirmatrelvir/ritonavir. A lower risk of the primary endpoint was observed in nirmatrelvir/ritonavir recipients compared with non-recipients [adjusted HR 0.56 (95% CI: 0.32–0.96); <jats:italic>p =</jats:italic> 0.035]. Nirmatrelvir/ritonavir recipients experienced a shorter duration of viral shedding [adjusted HR 3·70 (95%CI: 2.60–5.28); <jats:italic>p</jats:italic> &lt; 0.001) and faster viral load clearance versus non-recipients. Among the nirmatrelvir/ritonavir users, earlier initiation of nirmatrelvir/ritonavir within 5 days since COVID-19 diagnosis was related with shorter viral shedding time (adjusted HR 7.84 [95% CI: 3.28–18.76]; <jats:italic>p</jats:italic> &lt; 0.001) compared to late initiation. No patients reported serious adverse events during treatment.</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> Our findings support the early initiation of nirmatrelvir/ritonavir for high-risk patients with impaired kidney function. This could improve patient outcomes and shorten the viral shedding period.</jats:p>",
"alternative-id": [
"10.3389/fphar.2023.1147980"
],
"author": [
{
"affiliation": [],
"family": "Yan",
"given": "Jiayi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cai",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jieying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Mingli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Peiying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Bin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Che",
"given": "Xiajing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gu",
"given": "Leyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mou",
"given": "Shan",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
3,
22
]
],
"date-time": "2023-03-22T04:57:51Z",
"timestamp": 1679461071000
},
"deposited": {
"date-parts": [
[
2023,
3,
22
]
],
"date-time": "2023-03-22T04:57:55Z",
"timestamp": 1679461075000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"81970574 82170685"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
},
{
"DOI": "10.13039/100017950",
"award": [
"18ZXY001"
],
"doi-asserted-by": "publisher",
"name": "Shanghai Municipal Health Commission"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
23
]
],
"date-time": "2023-03-23T04:58:22Z",
"timestamp": 1679547502953
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
22
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
22
]
],
"date-time": "2023-03-22T00:00:00Z",
"timestamp": 1679443200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1147980/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
3,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
22
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"journal-title": "N. Engl. J. Med.",
"key": "B1",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1111/joim.13239",
"article-title": "Increased vulnerability to COVID-19 in chronic kidney disease",
"author": "Carlson",
"doi-asserted-by": "publisher",
"first-page": "166",
"journal-title": "J. Intern Med.",
"key": "B2",
"volume": "290",
"year": "2021"
},
{
"DOI": "10.1016/j.ajpath.2022.01.007",
"article-title": "Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in houston, Texas",
"author": "Christensen",
"doi-asserted-by": "publisher",
"first-page": "642",
"journal-title": "Am. J. Pathol.",
"key": "B3",
"volume": "192",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"article-title": "SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses",
"author": "Dejnirattisai",
"doi-asserted-by": "publisher",
"first-page": "467",
"journal-title": "Cell",
"key": "B4",
"volume": "185",
"year": "2022"
},
{
"key": "B5",
"volume-title": "Emergency use authorization for Paxlovid",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00997-x",
"article-title": "SARS-CoV-2 omicron variant: Recent progress and future perspectives",
"author": "Fan",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Signal Transduct. Target Ther.",
"key": "B6",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1053/j.ajkd.2020.09.003",
"article-title": "Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States",
"author": "Flythe",
"doi-asserted-by": "publisher",
"first-page": "190",
"journal-title": "Am. J. Kidney Dis.",
"key": "B7",
"volume": "77",
"year": "2021"
},
{
"key": "B8",
"volume-title": "Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27491",
"article-title": "Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "1255",
"journal-title": "J. Med. Virol.",
"key": "B9",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1161/JAHA.122.026143",
"article-title": "Renin-angiotensin system inhibitors in patients with COVID-19: A meta-analysis of randomized controlled trials led by the international society of hypertension",
"author": "Gnanenthiran",
"doi-asserted-by": "publisher",
"first-page": "e026143",
"journal-title": "J. Am. Heart Assoc.",
"key": "B10",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-03564-6",
"article-title": "Daily briefing: Omicron coronavirus variant puts scientists on alert",
"author": "Graham",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B11",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "B12",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Nat. Rev. Microbiol.",
"key": "B13",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1012",
"article-title": "Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET)",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "e206",
"journal-title": "Clin. Infect. Dis.",
"key": "B14",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.7326/0003-4819-150-9-200905050-00006",
"article-title": "A new equation to estimate glomerular filtration rate",
"author": "Levey",
"doi-asserted-by": "publisher",
"first-page": "604",
"journal-title": "Ann. Intern Med.",
"key": "B15",
"volume": "150",
"year": "2009"
},
{
"DOI": "10.1128/aac.01229-22",
"article-title": "Pharmacokinetics of nirmatrelvir and ritonavir in COVID-19 patients with end-stage renal disease on intermittent hemodialysis",
"author": "Lingscheid",
"doi-asserted-by": "publisher",
"first-page": "e0122922",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "B16",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2022.2109517",
"article-title": "Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave",
"author": "Lu",
"doi-asserted-by": "publisher",
"first-page": "2045",
"journal-title": "Emerg. Microbes Infect.",
"key": "B17",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n2713",
"article-title": "Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports",
"author": "Mahase",
"doi-asserted-by": "publisher",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "B18",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1186/s12951-021-01032-x",
"article-title": "The promising use of nano-molecular imprinted templates for improved SARS-CoV-2 detection, drug delivery and research",
"author": "Nahhas",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "J. Nanobiotechnology",
"key": "B19",
"volume": "19",
"year": "2021"
},
{
"article-title": "Diagnosis and treatment plan for COVID-19 (trial version 9)",
"first-page": "73",
"journal-title": "Int. J. Epidemiol. Infect. Dis.",
"key": "B20",
"volume": "49",
"year": "2022"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Science",
"key": "B21",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1093/ndt/gfaa271",
"article-title": "Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey",
"author": "Ozturk",
"doi-asserted-by": "publisher",
"first-page": "2083",
"journal-title": "Nephrol. Dial. Transpl.",
"key": "B22",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1126/science.abf1611",
"article-title": "SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model",
"author": "Qiao",
"doi-asserted-by": "publisher",
"first-page": "1374",
"journal-title": "Science",
"key": "B23",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1007/s40620-020-00875-1",
"article-title": "Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy",
"author": "Russo",
"doi-asserted-by": "publisher",
"first-page": "173",
"journal-title": "J. Nephrol.",
"key": "B24",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2688",
"article-title": "Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID-19, in subjects with renal impairment",
"author": "Toussi",
"doi-asserted-by": "publisher",
"first-page": "892",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "B25",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1007/s11684-021-0850-9",
"article-title": "Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "102",
"journal-title": "Front. Med.",
"key": "B26",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00838-8",
"article-title": "Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "2011",
"journal-title": "Lancet",
"key": "B27",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01530-6",
"article-title": "Protecting older people: A high priority during the COVID-19 pandemic",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "729",
"journal-title": "Lancet",
"key": "B28",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1186/s12951-021-01148-0",
"article-title": "Nanovesicles derived from bispecific CAR-T cells targeting the spike protein of SARS-CoV-2 for treating COVID-19",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "J. Nanobiotechnology",
"key": "B29",
"volume": "19",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1147980/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}