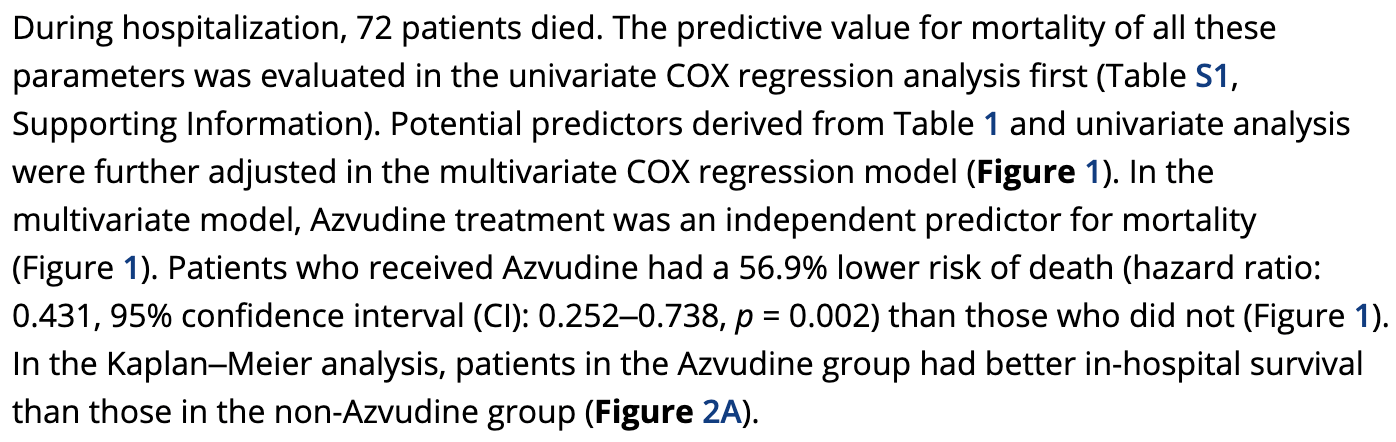
Azvudine for the Treatment of COVID‐19 in Pre‐Existing Cardiovascular Diseases: A Single‐Center, Real‐World Experience
et al., Advanced Science, doi:10.1002/advs.202306050, Mar 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 351 hospitalized COVID-19 patients with pre-existing cardiovascular diseases in China, showing lower mortality with azvudine treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 81.1% lower, HR 0.19, p < 0.001, treatment 90, control 90, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of death, 56.9% lower, HR 0.43, p = 0.002, treatment 106, control 245, adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Wu et al., 27 Mar 2024, retrospective, China, peer-reviewed, 7 authors, average treatment delay 2.0 days.
Contact: chenmanhua@zxhospital.com, zhanghongda@fuwai.com.
Azvudine for the Treatment of COVID‐19 in Pre‐Existing Cardiovascular Diseases: A Single‐Center, Real‐World Experience
Advanced Science, doi:10.1002/advs.202306050
COVID-19 can lead to adverse outcomes in patients with pre-existing diseases. Azvudine has been approved for treating COVID-19 in China, but the real-world data is limited. It is aimed to investigate the efficacy of Azvudine in patients with COVID-19 and pre-existing cardiovascular diseases. Patients with confirmed COVID-19 and pre-existing cardiovascular diseases are retrospectively enrolled. The primary outcome is all-cause death during hospitalization. Overall, 351 patients are included, with a median age of 74 years, and 44% are female. 212 (60.6%) patients are severe cases. Azvudine is used in 106 (30.2%) patients and not in 245 (69.8%). 72 patients died during hospitalization. After multivariate adjustment, patients who received Azvudine a lower risk of all-cause death (hazard ratio: 0.431; 95% confidence interval: 0.252-0.738; p = 0.002) than controls. Azvudine therapy is also associated with lower risks of shock and acute kidney injury. For sensitivity analysis in the propensity score-matched cohort (n = 90 for each group), there is also a significant difference in all-cause death between the two groups (hazard ratio: 0.189; 95% confidence interval: 0.071-0.498; p < 0.001). This study indicated that Azvudine therapy is associated with better outcomes in COVID-19 patients with pre-existing cardiovascular diseases.
Supporting Information Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest The authors declare no conflict of interest.
Author Contributions
References
Deng, Li, Sun, Jin, Zhou et al., None, J. Med. Virol
Dian, Meng, Sun, Deng, Zeng, None, J Infect
Huang, Fei, Li, Xu, Xie et al., None, Int J Infect Dis
Huang, Wang, Li, Ren, Zhao et al., None, Lancet
Nhco, New coronavirus pneumonia prevention and control program
Ren, Luo, Yu, Song, Liang et al., None, Adv. Sci. (Weinh)
Sun, Jin, Dian, Shen, Zeng et al., None, EClini-calMedicine
Wenham, None, BMJ Clin. Res. Ed
Wise, None, BMJ Clin. Res. Ed
Yang, Wang, None, Eur. J. Med. Chem
Yu, Chang, None, Innovation (Camb)
Yu, Chang, None, Signal Transduct Target Ther
Zarocostas, None, Lancet
Zhang, Li, Wang, Liu, Lu et al., None, Signal. Transduct. Target. Ther
Zhang, Wang, Zhang, Zhou, Deng et al., None, Glob. Health Med
DOI record:
{
"DOI": "10.1002/advs.202306050",
"ISSN": [
"2198-3844",
"2198-3844"
],
"URL": "http://dx.doi.org/10.1002/advs.202306050",
"abstract": "<jats:title>Abstract</jats:title><jats:p>COVID‐19 can lead to adverse outcomes in patients with pre‐existing diseases. Azvudine has been approved for treating COVID‐19 in China, but the real‐world data is limited. It is aimed to investigate the efficacy of Azvudine in patients with COVID‐19 and pre‐existing cardiovascular diseases. Patients with confirmed COVID‐19 and pre‐existing cardiovascular diseases are retrospectively enrolled. The primary outcome is all‐cause death during hospitalization. Overall, 351 patients are included, with a median age of 74 years, and 44% are female. 212 (60.6%) patients are severe cases. Azvudine is used in 106 (30.2%) patients and not in 245 (69.8%). 72 patients died during hospitalization. After multivariate adjustment, patients who received Azvudine a lower risk of all‐cause death (hazard ratio: 0.431; 95% confidence interval: 0.252–0.738; <jats:italic>p</jats:italic> = 0.002) than controls. Azvudine therapy is also associated with lower risks of shock and acute kidney injury. For sensitivity analysis in the propensity score‐matched cohort (<jats:italic>n</jats:italic> = 90 for each group), there is also a significant difference in all‐cause death between the two groups (hazard ratio: 0.189; 95% confidence interval: 0.071–0.498; <jats:italic>p</jats:italic> < 0.001). This study indicated that Azvudine therapy is associated with better outcomes in COVID‐19 patients with pre‐existing cardiovascular diseases.</jats:p>",
"alternative-id": [
"10.1002/advs.202306050"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-08-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-03-27"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "Wu",
"given": "Liu",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "He",
"given": "Zhong‐Han",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "Huang",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "Guo",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "Li",
"given": "Xu‐Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases Chinese Academy of Medical Sciences & Peking Union Medical College Beijing 100037 China"
}
],
"family": "Zhang",
"given": "Hong‐Da",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College Huazhong University of Science and Technology Wuhan 430014 China"
}
],
"family": "Chen",
"given": "Man‐Hua",
"sequence": "additional"
}
],
"container-title": "Advanced Science",
"container-title-short": "Advanced Science",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T05:40:33Z",
"timestamp": 1711604433000
},
"deposited": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T05:40:41Z",
"timestamp": 1711604441000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"82000064;81900334"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T01:18:17Z",
"timestamp": 1711675097807
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3,
27
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
27
]
],
"date-time": "2024-03-27T00:00:00Z",
"timestamp": 1711497600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/advs.202306050",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
3,
27
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
27
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_1_1"
},
{
"DOI": "10.1016/S0140-6736(23)01003-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"author": "Wenham C.",
"journal-title": "BMJ Clin. Res. Ed.",
"key": "e_1_2_10_3_1",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1136/bmj.p1041",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.35772/ghm.2022.01063",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"key": "e_1_2_10_6_1",
"unstructured": "Prevention CCFDCa The National COVID‐19 Infection Status.https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202306/t20230611_266656.htm.2023. June."
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1002/advs.202001435",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1002/jmv.28756",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"key": "e_1_2_10_12_1",
"unstructured": "China NHCo New coronavirus pneumonia prevention and control program (the tenth edition).http://www.nhc.gov.cn/xcs/zhengcwj/202301/bdc1ff75feb94934ae1dade176d30936.shtml.2023. January."
},
{
"DOI": "10.1038/s41392-020-00351-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"author": "Dian Y.",
"first-page": "27",
"journal-title": "J Infect.",
"key": "e_1_2_10_15_1",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2021.01.009",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1016/S0140-6736(22)00101-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/advs.202306050"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Physics and Astronomy",
"General Engineering",
"Biochemistry, Genetics and Molecular Biology (miscellaneous)",
"General Materials Science",
"General Chemical Engineering",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "Azvudine for the Treatment of COVID‐19 in Pre‐Existing Cardiovascular Diseases: A Single‐Center, Real‐World Experience",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
