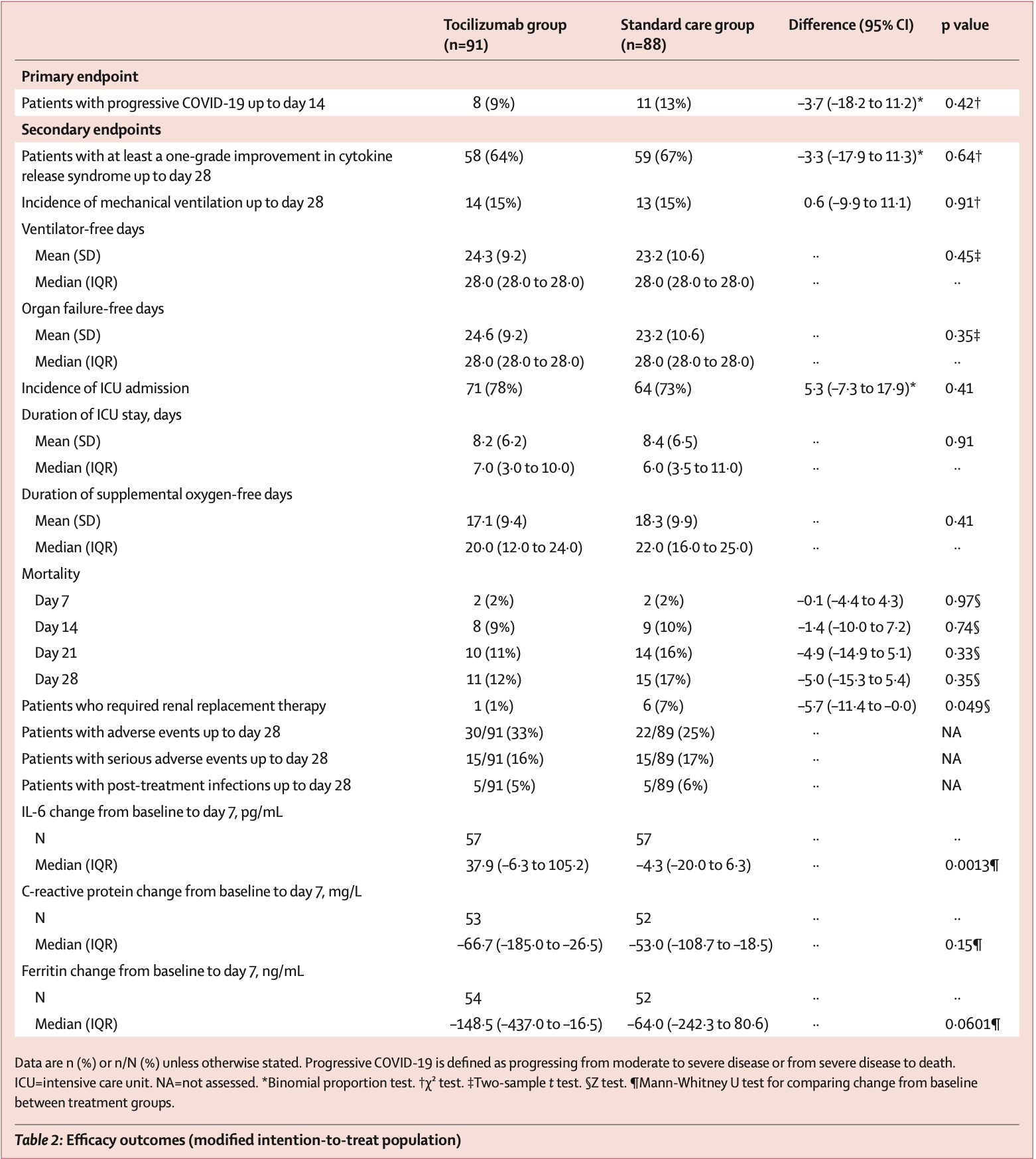
Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00081-3, COVINTOC, CTRI/2020/05/025369, May 2021
RCT 180 hospitalized patients with moderate to severe COVID-19 in India showing no significant difference in disease progression with tocilizumab.
|
risk of death, 29.1% lower, RR 0.71, p = 0.40, treatment 11 of 91 (12.1%), control 15 of 88 (17.0%), NNT 20.
|
|
risk of mechanical ventilation, 4.1% higher, RR 1.04, p = 1.00, treatment 14 of 91 (15.4%), control 13 of 88 (14.8%).
|
|
risk of progression, 29.7% lower, RR 0.70, p = 0.47, treatment 8 of 91 (8.8%), control 11 of 88 (12.5%), NNT 27.
|
|
risk of ICU admission, 7.3% higher, RR 1.07, p = 0.49, treatment 71 of 91 (78.0%), control 64 of 88 (72.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Soin et al., 31 May 2021, Randomized Controlled Trial, India, peer-reviewed, median age 56.0, 20 authors, study period 30 May, 2020 - 31 August, 2020, trial CTRI/2020/05/025369 (COVINTOC).
Contact: avisoin1@gmail.com.
Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00081-3
Background Global randomised controlled trials of the anti-IL-6 receptor antibody tocilizumab in patients admitted to hospital with COVID-19 have shown conflicting results but potential decreases in time to discharge and burden on intensive care. Tocilizumab reduced progression to mechanical ventilation and death in a trial population enriched for racial and ethnic minorities. We aimed to investigate whether tocilizumab treatment could prevent COVID-19 progression in the first multicentre randomised controlled trial of tocilizumab done entirely in a lower-middle-income country.
Methods COVINTOC is an open-label, multicentre, randomised, controlled, phase 3 trial done at 12 public and private hospitals across India. Adults (aged ≥18 years) admitted to hospital with moderate to severe COVID-19 (Indian Ministry of Health grading) confirmed by positive SARS-CoV-2 PCR result were randomly assigned (1:1 block randomisation) to receive tocilizumab 6 mg/kg plus standard care (the tocilizumab group) or standard care alone (the standard care group). The primary endpoint was progression of COVID-19 (from moderate to severe or from severe to death) up to day 14 in the modified intention-to-treat population of all participants who had at least one postbaseline assessment for the primary endpoint. Safety was assessed in all randomly assigned patients. The trial is completed and registered with the Clinical Trials Registry India (CTRI/2020/05/025369). Findings 180 patients were recruited between May 30, 2020, and Aug 31, 2020, and randomly assigned to the tocilizumab group (n=90) or the standard care group (n=90). One patient randomly assigned to the standard care group inadvertently received tocilizumab at baseline and was included in the tocilizumab group for all analyses. One patient randomly assigned to the standard care group withdrew consent after the baseline visit and did not receive any study medication and was not included in the modified intention-to-treat population but was still included in safety analyses. 75 (82%) of 91 in the tocilizumab group and 68 (76%) of 89 in the standard care group completed 28 days of follow-up. Progression of COVID-19 up to day 14 occurred in eight (9%) of 91 patients in the tocilizumab group and 11 (13%) of 88 in the standard care group (difference -3•71 [95% CI -18•23 to 11•19]; p=0•42). 33 (36%) of 91 patients in the tocilizumab group and 22 (25%) of 89 patients in the standard care group had adverse events; 18 (20%) and 15 (17%) had serious adverse events. The most common adverse event was acute respiratory distress syndrome, reported in seven (8%) patients in each group. Grade 3 adverse events were reported in two (2%) patients in the tocilizumab group and five (6%) patients in the standard care group. There were no grade 4 adverse events. Serious adverse events were reported in 18 (20%) patients in the tocilizumab group and 15 (17%) in the standard care group; 13 (14%) and 15 (17%) patients died during the..
References
Antwi-Amoabeng, Kanji, Ford, Beutler, Riddle et al., Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review, J Med Virol
Aziz, Fatima, Assaly, Elevated interleukin-6 and severe COVID-19: a meta-analysis, J Med Virol
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Conti, Ronconi, Caraffa, Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies, J Biol Regul Homeost Agents
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hermine, Mariette, Tharaux, Resche-Rigon, Porcher et al., Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial, JAMA Intern Med
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Lee, Santomasso, Locke, ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells, Biol Blood Marrow Transplant
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, J Med Virol
Netea, Rovina, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host Microbe
Nishimoto, Terao, Mima, Nakahara, Takagi et al., Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease, Blood
Rosas, Bräu, Waters, Tocilizumab in hospitalized patients with COVID-19 pneumonia, medRxiv, doi:10.1101/2020.08.27.20183442
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Rubbert-Roth, Furst, Nebesky, Berber, A review of recent advances using tocilizumab in the treatment of rheumatic diseases, Rheumatol Ther
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Sharma, Chowdhury, Challenges and dilemmas of operationalizing COVID-19 clinical research in India
Stone, Frigault, Nj, Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci
Zhu, Pang, Ji, Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis, J Med Virol, doi:10.1002/jmv.26085
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00081-3",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00081-3",
"alternative-id": [
"S2213260021000813"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00081-3"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(21)00127-2"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Soin",
"given": "Arvinder S",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Kuldeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhary",
"given": "Narendra S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Pooja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehta",
"given": "Yatin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kataria",
"given": "Sushila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Govil",
"given": "Deepak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deswal",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaudhry",
"given": "Dhruva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Pawan Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agarwal",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Suresh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sangle",
"given": "Shashikala A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chawla",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Narreddy",
"given": "Suneetha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pandit",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mishra",
"given": "Vipul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goel",
"given": "Manoj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramanan",
"given": "Athimalaipet V",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
3,
7
]
],
"date-time": "2021-03-07T17:21:18Z",
"timestamp": 1615137678000
},
"deposited": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T06:42:12Z",
"timestamp": 1683009732000
},
"indexed": {
"date-parts": [
[
2025,
5,
26
]
],
"date-time": "2025-05-26T21:42:44Z",
"timestamp": 1748295764255
},
"is-referenced-by-count": 188,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021000813?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021000813?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "511-521",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
5
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary report",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00081-3_bib1",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00081-3_bib2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00081-3_bib3",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"article-title": "Complex immune dysregulation in COVID-19 patients with severe respiratory failure",
"author": "Giamarellos-Bourboulis",
"doi-asserted-by": "crossref",
"first-page": "992",
"journal-title": "Cell Host Microbe",
"key": "10.1016/S2213-2600(21)00081-3_bib4",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00081-3_bib5",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00081-3_bib6",
"volume": "323",
"year": "2020"
},
{
"article-title": "Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies",
"author": "Conti",
"first-page": "327",
"journal-title": "J Biol Regul Homeost Agents",
"key": "10.1016/S2213-2600(21)00081-3_bib7",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25948",
"article-title": "Elevated interleukin-6 and severe COVID-19: a meta-analysis",
"author": "Aziz",
"doi-asserted-by": "crossref",
"first-page": "2283",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00081-3_bib8",
"volume": "92",
"year": "2020"
},
{
"article-title": "Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis",
"author": "Zhu",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00081-3_bib9",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China",
"author": "Ruan",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(21)00081-3_bib10",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1007/s40744-018-0102-x",
"article-title": "A review of recent advances using tocilizumab in the treatment of rheumatic diseases",
"author": "Rubbert-Roth",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Rheumatol Ther",
"key": "10.1016/S2213-2600(21)00081-3_bib11",
"volume": "5",
"year": "2018"
},
{
"DOI": "10.1002/jmv.26038",
"article-title": "Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review",
"author": "Antwi-Amoabeng",
"doi-asserted-by": "crossref",
"first-page": "2516",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00081-3_bib12",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "10970",
"journal-title": "Proc Natl Acad Sci USA",
"key": "10.1016/S2213-2600(21)00081-3_bib13",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25801",
"article-title": "Tocilizumab treatment in COVID-19: a single center experience",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "814",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(21)00081-3_bib14",
"volume": "92",
"year": "2020"
},
{
"article-title": "Tocilizumab in hospitalized patients with COVID-19 pneumonia",
"author": "Rosas",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(21)00081-3_bib15",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with Covid-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00081-3_bib16",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028836",
"article-title": "Efficacy of tocilizumab in patients hospitalized with Covid-19",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "2333",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00081-3_bib17",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"article-title": "Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial",
"author": "Hermine",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(21)00081-3_bib18",
"volume": "181",
"year": "2021"
},
{
"author": "Sharma",
"key": "10.1016/S2213-2600(21)00081-3_bib19"
},
{
"DOI": "10.1097/CM9.0000000000000819",
"article-title": "Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7)",
"doi-asserted-by": "crossref",
"first-page": "1087",
"journal-title": "Chin Med J",
"key": "10.1016/S2213-2600(21)00081-3_bib21",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1016/j.bbmt.2018.12.758",
"article-title": "ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "625",
"journal-title": "Biol Blood Marrow Transplant",
"key": "10.1016/S2213-2600(21)00081-3_bib22",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1182/blood-2008-05-155846",
"article-title": "Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease",
"author": "Nishimoto",
"doi-asserted-by": "crossref",
"first-page": "3959",
"journal-title": "Blood",
"key": "10.1016/S2213-2600(21)00081-3_bib24",
"volume": "112",
"year": "2008"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021000813"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}