Efficacy and Safety of Remdesivir in People With Impaired Kidney Function Hospitalized for Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae333, REDPINE, NCT04745351, Jun 2024
RCT 243 hospitalized COVID-19 patients with acute kidney injury, chronic kidney disease, or kidney failure showing no significant difference in all-cause mortality or invasive mechanical ventilation with remdesivir. The lower mortality at day 29 (without statistical significance) disappeared at day 60, consistent with remdesivir studies overall.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
|
risk of death, 5.8% higher, RR 1.06, p = 0.89, treatment 55 of 166 (33.1%), control 26 of 83 (31.3%), last followup, registry.
|
|
risk of death, 0.1% higher, RR 1.00, p = 1.00, treatment 51 of 163 (31.3%), control 25 of 80 (31.2%), day 60.
|
|
risk of death, 17.0% lower, HR 0.83, p = 0.39, treatment 41 of 163 (25.2%), control 23 of 80 (28.7%), NNT 28, Cox proportional hazards, day 29.
|
|
risk of death/intubation, 18.0% lower, HR 0.82, p = 0.61, treatment 48 of 163 (29.4%), control 26 of 80 (32.5%), NNT 33, Cox proportional hazards, day 29.
|
|
clinical status, 16.0% lower, OR 0.84, p = 0.50, treatment 163, control 80, day 29, RR approximated with OR.
|
|
clinical status, 5.0% higher, OR 1.05, p = 0.85, treatment 163, control 80, day 15, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Sise et al., 24 Jun 2024, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 69.0, 24 authors, study period March 2021 - March 2022, trial NCT04745351 (history) (REDPINE).
Contact: msise@mgb.org.
Efficacy and Safety of Remdesivir in People With Impaired Kidney Function Hospitalized for Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial
doi:10.1093/cid/ciae333/7697980
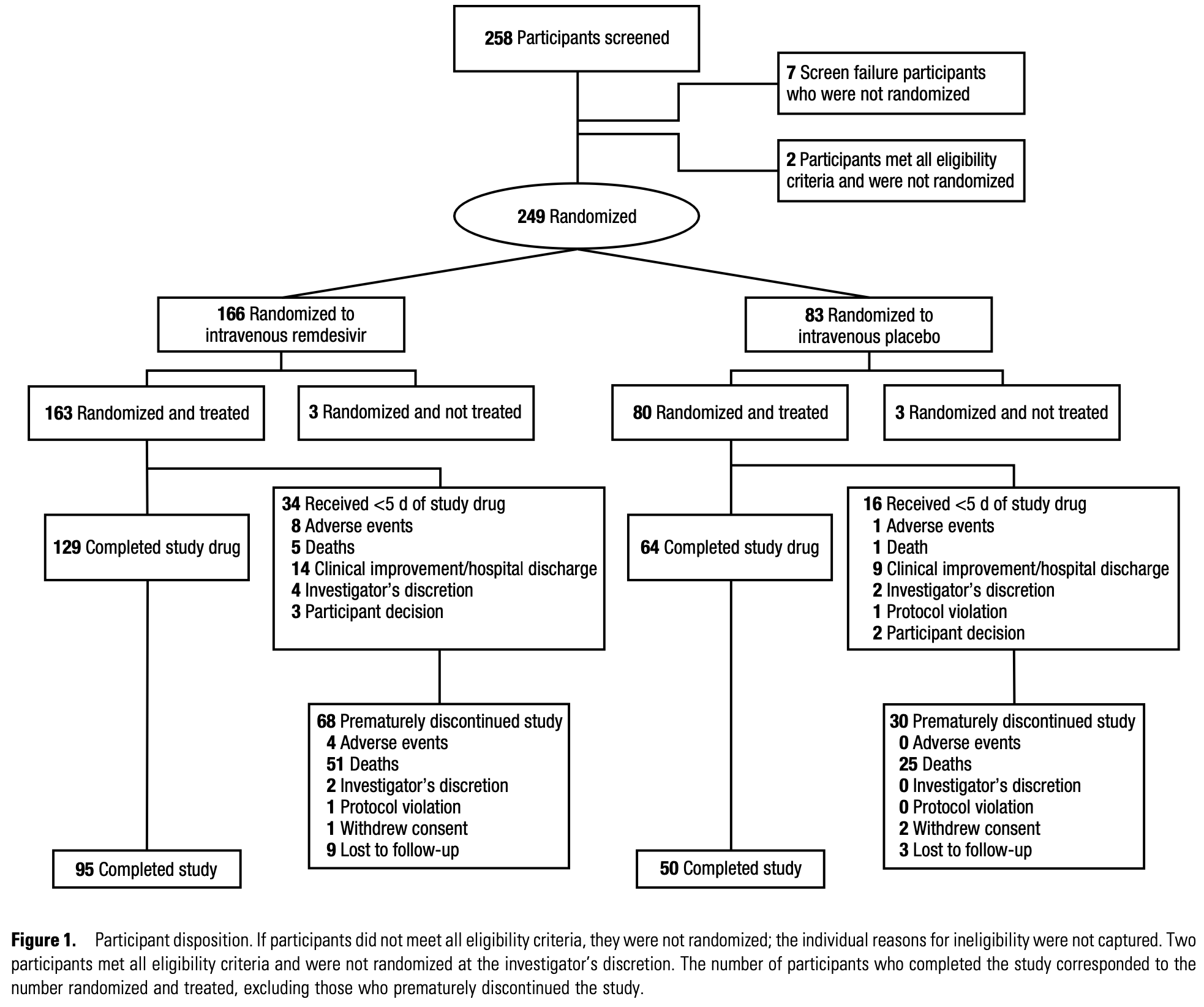
Background. Few antiviral therapies have been studied in patients with coronavirus disease 2019 (COVID-19) and kidney impairment. Herein, the efficacy, safety, and pharmacokinetics of remdesivir, its metabolites, and sulfobutylether-β-cyclodextrin excipient were evaluated in hospitalized patients with COVID-19 and severe kidney impairment. Methods. In REDPINE, a phase 3, randomized, double-blind, placebo-controlled study, participants aged ≥12 years hospitalized for COVID-19 pneumonia with acute kidney injury, chronic kidney disease, or kidney failure were randomized 2:1 to receive intravenous remdesivir (200 mg on day 1; 100 mg daily up to day 5) or placebo (enrollment from March 2021 to March 2022). The primary efficacy end point was the composite of the all-cause mortality rate or invasive mechanical ventilation rate through day 29. Safety was evaluated through day 60. Results. Although enrollment concluded early, 243 participants were enrolled and treated (remdesivir, n = 163; placebo, n = 80). At baseline, 90 participants (37.0%) had acute kidney injury (remdesivir, n = 60; placebo, n = 30), 64 (26.3%) had chronic kidney disease (remdesivir, n = 44; placebo, n = 20), and 89 (36.6%) had kidney failure (remdesivir, n = 59; placebo, n = 30); and 31 (12.8%) were vaccinated against COVID-19. Composite all-cause mortality or invasive mechanical ventilation rates through day 29 were 29.4% and 32.5% in the remdesivir and placebo group, respectively (P = .61). Treatment-emergent adverse events were reported in 80.4% for remdesivir versus 77.5% for placebo, and serious adverse events in 50.3% versus 50.0%, respectively. Pharmacokinetic plasma exposure to remdesivir was not affected by kidney function. Conclusions. Although the study was underpowered, no significant difference in efficacy was observed between treatment groups. REDPINE demonstrated that remdesivir is safe in patients with COVID-19 and severe kidney impairment.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes Acknowledgments. Medical writing and editorial support were provided by Laura Watts, PhD, of Lumanity Communications Inc. (Yardley, Pennsylvania, USA), and were funded by Gilead Sciences. Investigators thank the patients and their families for their participation Author Contributions. M. E. S. and Y. K. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M. E. S., K. R.
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-final report, N Engl J Med
Bell, Campbell, Lambourg, The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland, J Am Soc Nephrol
Chokkalingam, Hayden, Goldman, Association of remdesivir treatment with mortality among hospitalized adults with COVID-19 in the United States, JAMA Netw Open
Council, Eracoda, Group, Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA, Nephrol Dial Transplant
Estcourt, Callum, Convalescent plasma for COVID-19-making sense of the inconsistencies, N Engl J Med
Goldman, Lye, Hui, Remdesivir for 5 or 10 days in patients with severe COVID-19, N Engl J Med
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe COVID-19 in outpatients, N Engl J Med
Hoover, Alcorn, Jr, Lawrence, Clinical pharmacokinetics of sulfobutylether-β-cyclodextrin in patients with varying degrees of renal impairment, J Clin Pharmacol
Humeniuk, Mathias, Kirby, Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor, Clin Pharmacokinet
Kellum, Lameire, Aspelin, KDIGO clinical practice guideline for acute kidney injury, Kidney Int Suppl
Kiser, Fish, Aquilante, Evaluation of sulfobutyletherβ-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy, Crit Care
Levey, Stevens, Schmid, A new equation to estimate glomerular filtration rate, Ann Intern Med
Luke, Tomaszewski, Damle, Schlamm, Review of the basic and clinical pharmacology of sulfobutylether-β-cyclodextrin (SBECD), J Pharm Sci
Luke, Wood, Tomaszewski, Damle, Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis, Nephrol Dial Transplant
Marra, Smolders, El-Sherif, Recommendations for dosing of repurposed COVID-19 medications in patients with renal and hepatic impairment, Drugs R D
Mozaffari, Chandak, Gottlieb, Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants, Open Forum Infect Dis
Mozaffari, Chandak, Zhang, July 2024 hospital all-cause mortality in a large multicenter observational cohort, doi:10.1093/cid/ciae333/7697980bygueston25
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant, N Engl J Med
Wang, Zhang, Comment about the safety of intravenous voriconazole formulated with sulfobutylether beta-cyclodextrin, Exp Opin Drug Saf
DOI record:
{
"DOI": "10.1093/cid/ciae333",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae333",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Few antiviral therapies have been studied in patients with coronavirus disease 2019 (COVID-19) and kidney impairment. Herein, the efficacy, safety, and pharmacokinetics of remdesivir, its metabolites, and sulfobutylether-β-cyclodextrin excipient were evaluated in hospitalized patients with COVID-19 and severe kidney impairment.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In REDPINE, a phase 3, randomized, double-blind, placebo-controlled study, participants aged ≥12 years hospitalized for COVID-19 pneumonia with acute kidney injury, chronic kidney disease, or kidney failure were randomized 2:1 to receive intravenous remdesivir (200 mg on day 1; 100 mg daily up to day 5) or placebo (enrollment from March 2021 to March 2022). The primary efficacy end point was the composite of the all-cause mortality rate or invasive mechanical ventilation rate through day 29. Safety was evaluated through day 60.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Although enrollment concluded early, 243 participants were enrolled and treated (remdesivir, n = 163; placebo, n = 80). At baseline, 90 participants (37.0%) had acute kidney injury (remdesivir, n = 60; placebo, n = 30), 64 (26.3%) had chronic kidney disease (remdesivir, n = 44; placebo, n = 20), and 89 (36.6%) had kidney failure (remdesivir, n = 59; placebo, n = 30); and 31 (12.8%) were vaccinated against COVID-19. Composite all-cause mortality or invasive mechanical ventilation rates through day 29 were 29.4% and 32.5% in the remdesivir and placebo group, respectively (P = .61). Treatment-emergent adverse events were reported in 80.4% for remdesivir versus 77.5% for placebo, and serious adverse events in 50.3% versus 50.0%, respectively. Pharmacokinetic plasma exposure to remdesivir was not affected by kidney function.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Although the study was underpowered, no significant difference in efficacy was observed between treatment groups. REDPINE demonstrated that remdesivir is safe in patients with COVID-19 and severe kidney impairment.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>EudraCT 2020-005416-22; Clinical Trials.gov NCT04745351.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of Nephrology, Massachusetts General Hospital , Boston, Massachusetts , USA"
}
],
"family": "Sise",
"given": "Meghan E",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Fight Infections Foundation, Service of Infectious Diseases, Hospital Universitari Germans Trias i Pujol , Badalona , Spain"
}
],
"family": "Santos",
"given": "Jose Ramon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Swedish Center for Research and Innovation, Providence Swedish Medical Center , Seattle, Washington , USA"
},
{
"name": "Division of Allergy and Infectious Diseases, University of Washington , Seattle, Washington , USA"
}
],
"family": "Goldman",
"given": "Jason D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Providence Medical Research Center, Providence Inland Northwest Health , Spokane, Washington , USA"
}
],
"family": "Tuttle",
"given": "Katherine R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Divisions of Nephrology and Pulmonary, Critical Care, and Sleep Medicine, University of New Mexico Hospital , Albuquerque, New Mexico , USA"
}
],
"family": "Teixeira",
"given": "J Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonary Associates Research, Ascension Providence , Mobile, Alabama , USA"
}
],
"family": "Seibert",
"given": "Allan F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Koullias",
"given": "Yiannis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Llewellyn",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Regan",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Zhao",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Huang",
"given": "Hailin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Hyland",
"given": "Robert H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Osinusi",
"given": "Anu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Winter",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gilead Sciences , Foster City, California , USA"
}
],
"family": "Humeniuk",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California , San Francisco, California , USA"
}
],
"family": "Hulter",
"given": "Henry N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Baylor University Medical Center , Dallas, Texas , USA"
},
{
"name": "Baylor Scott & White Research Institute , Dallas, Texas , USA"
}
],
"family": "Gottlieb",
"given": "Robert L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Tulane University School of Medicine , New Orleans, Louisiana , USA"
}
],
"family": "Fusco",
"given": "Dahlene N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Centro Hospitalar de Lisboa Ocidental , Lisbon , Portugal"
},
{
"name": "NOVA Medical School , Lisbon , Portugal"
}
],
"family": "Birne",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Mayo Clinic College of Medicine and Science , Jacksonville, Florida , USA"
}
],
"family": "Stancampiano",
"given": "Fernando F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Mayo Clinic College of Medicine and Science , Jacksonville, Florida , USA"
}
],
"family": "Libertin",
"given": "Claudia R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, Weill Cornell Medicine , New York, New York , USA"
}
],
"family": "Small",
"given": "Catherine B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, Weill Cornell Medicine , New York, New York , USA"
}
],
"family": "Plate",
"given": "Markus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Liver Studies, King's College Hospital , London , United Kingdom"
}
],
"family": "McPhail",
"given": "Mark J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ballesteros",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Birne",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malheiro",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Gil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Correia",
"given": "João Paulo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vida",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Andre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carujo",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Moncef Belhassen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandez",
"given": "Jordi Carratala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abelenda-Alonso",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruzado",
"given": "Josep M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rombauts",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandoval",
"given": "Diego A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deltoro",
"given": "Miguel Garcia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gimeno",
"given": "Fransesc Puchades",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Muñoz",
"given": "Neus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roma",
"given": "Maria Martínez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallego",
"given": "Juan Horcajada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pablo",
"given": "Castañeda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silvia",
"given": "Padilla Urrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sergio",
"given": "Rial Crestelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "David",
"given": "Santos Fernandez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jose",
"given": "Ramon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benet",
"given": "Susanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benítez",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bracke",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chamorro",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "España",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graterol",
"given": "Fredzzia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LLadós",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateu",
"given": "Lourdes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paredes",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rebollo",
"given": "Boris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero",
"given": "Alba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soldevila",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abad",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chamorro",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "José",
"given": "Alba San",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viladomiu",
"given": "Alex Soriano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McPhail",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Medjeral-Thomas",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lobo",
"given": "Suzana Margareth Ajeje",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abolnik",
"given": "Igor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acharya",
"given": "Anjali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Leland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellovich",
"given": "Keith A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burton",
"given": "Mary Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cameron",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Criner",
"given": "Gerard J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Criner",
"given": "Lii-Yoong H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lambert",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rashid",
"given": "Marium",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shore-Brown",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diaz",
"given": "George A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dougherty",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erdmann",
"given": "Nathaniel B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fusco",
"given": "Dahlene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldman",
"given": "Jason D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berrington",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Logar",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vadivel",
"given": "Nidyanandh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Everett",
"given": "Allison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maria Lourdes",
"given": "Gonzalez Suarez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gottlieb",
"given": "Robert L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berhe",
"given": "Mezgebe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colbert",
"given": "Gates",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hebert",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehta",
"given": "Ankit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spak",
"given": "Cedric W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estrada",
"given": "Lorie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vargas",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choe",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pham",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mason",
"given": "L Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tallmadge",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Braddom",
"given": "Ariana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicholas",
"given": "Maldonado",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jamil",
"given": "Aayla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McAllister",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guerra",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sam",
"given": "Teena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solis",
"given": "Edilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gotur",
"given": "Deepa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goyal",
"given": "Munish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koraishy",
"given": "Farrukh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laurence",
"given": "Brett",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malhotra",
"given": "Vinay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manrique",
"given": "Luis A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKinnell",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McMahon",
"given": "Blaithin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Campbell",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morse",
"given": "Caryn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navarro",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ostrosky",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grimes",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernandez",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mammadova",
"given": "Mehriban",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Umana",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pusch",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanyal",
"given": "Arun J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schrager",
"given": "Harry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mallada",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seibert",
"given": "Allan F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siegel",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sise",
"given": "Meghan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slim",
"given": "Jihad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Small",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sriram",
"given": "Peruvemba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stancampiano",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Joao Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Apodaca",
"given": "Krystle D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harkins",
"given": "Michelle S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunningham",
"given": "Amy G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuttle",
"given": "Katherine R",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the REDPINE Investigators",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T16:55:32Z",
"timestamp": 1719248132000
},
"deposited": {
"date-parts": [
[
2024,
7,
22
]
],
"date-time": "2024-07-22T10:11:32Z",
"timestamp": 1721643092000
},
"funder": [
{
"DOI": "10.13039/100005564",
"doi-asserted-by": "publisher",
"name": "Gilead Sciences"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
22
]
],
"date-time": "2024-07-22T10:40:17Z",
"timestamp": 1721644817502
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
24
]
],
"date-time": "2024-06-24T00:00:00Z",
"timestamp": 1719187200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae333/58607179/ciae333.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae333/58607179/ciae333.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
6,
24
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
24
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1093/ndt/gfaa314",
"article-title": "Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA",
"author": "ERA-EDTA Council, ERACODA Working Group",
"doi-asserted-by": "crossref",
"first-page": "87",
"journal-title": "Nephrol Dial Transplant",
"key": "2024072210104968200_ciae333-B1",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1681/ASN.2022010046",
"article-title": "The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland",
"author": "Bell",
"doi-asserted-by": "crossref",
"first-page": "677",
"journal-title": "J Am Soc Nephrol",
"key": "2024072210104968200_ciae333-B2",
"volume": "33",
"year": "2022"
},
{
"DOI": "10.1007/s40268-020-00333-0",
"article-title": "Recommendations for dosing of repurposed COVID-19 medications in patients with renal and hepatic impairment",
"author": "Marra",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Drugs R D",
"key": "2024072210104968200_ciae333-B3",
"volume": "21",
"year": "2021"
},
{
"author": "VEKLURY® (remdesivir) [prescribing information]",
"key": "2024072210104968200_ciae333-B4",
"year": "2022"
},
{
"DOI": "10.1007/s40262-021-00984-5",
"article-title": "Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor",
"author": "Humeniuk",
"doi-asserted-by": "crossref",
"first-page": "569",
"journal-title": "Clin Pharmacokinet",
"key": "2024072210104968200_ciae333-B5",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1186/s13054-015-0753-8",
"article-title": "Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy",
"author": "Kiser",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "Crit Care",
"key": "2024072210104968200_ciae333-B6",
"volume": "19",
"year": "2015"
},
{
"DOI": "10.7326/0003-4819-150-9-200905050-00006",
"article-title": "A new equation to estimate glomerular filtration rate",
"author": "Levey",
"doi-asserted-by": "crossref",
"first-page": "604",
"journal-title": "Ann Intern Med",
"key": "2024072210104968200_ciae333-B7",
"volume": "150",
"year": "2009"
},
{
"article-title": "KDIGO clinical practice guideline for acute kidney injury",
"author": "Kellum",
"first-page": "1",
"journal-title": "Kidney Int Suppl",
"key": "2024072210104968200_ciae333-B8",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 days in patients with severe COVID-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N Engl J Med",
"key": "2024072210104968200_ciae333-B9",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "2024072210104968200_ciae333-B10",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2022.44505",
"article-title": "Association of remdesivir treatment with mortality among hospitalized adults with COVID-19 in the United States",
"author": "Chokkalingam",
"doi-asserted-by": "crossref",
"first-page": "e2244505",
"journal-title": "JAMA Netw Open",
"key": "2024072210104968200_ciae333-B11",
"volume": "5",
"year": "2022"
},
{
"article-title": "Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants",
"author": "Mozaffari",
"first-page": "ofad482",
"key": "2024072210104968200_ciae333-B12",
"volume-title": "Open Forum Infect Dis",
"year": "2023"
},
{
"article-title": "Fact sheet for health care providers. Emergency use authorization (EUA) of VEKLURY® (remdesivir)",
"author": "Gilead Sciences",
"key": "2024072210104968200_ciae333-B13"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N Engl J Med",
"key": "2024072210104968200_ciae333-B14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMe2204332",
"article-title": "Convalescent plasma for COVID-19—making sense of the inconsistencies",
"author": "Estcourt",
"doi-asserted-by": "crossref",
"first-page": "1753",
"journal-title": "N Engl J Med",
"key": "2024072210104968200_ciae333-B15",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe COVID-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2024072210104968200_ciae333-B16",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab875",
"article-title": "Remdesivir treatment in hospitalized patients with coronavirus disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort",
"author": "Mozaffari",
"doi-asserted-by": "crossref",
"first-page": "e450",
"journal-title": "Clin Infect Dis",
"key": "2024072210104968200_ciae333-B17",
"volume": "75",
"year": "2022"
},
{
"author": "European Medicines Agency",
"key": "2024072210104968200_ciae333-B18"
},
{
"DOI": "10.1002/jps.22109",
"article-title": "Review of the basic and clinical pharmacology of sulfobutylether-β-cyclodextrin (SBECD)",
"author": "Luke",
"doi-asserted-by": "crossref",
"first-page": "3291",
"journal-title": "J Pharm Sci",
"key": "2024072210104968200_ciae333-B19",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.1093/ndt/gfr472",
"article-title": "Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis",
"author": "Luke",
"doi-asserted-by": "crossref",
"first-page": "1207",
"journal-title": "Nephrol Dial Transplant",
"key": "2024072210104968200_ciae333-B20",
"volume": "27",
"year": "2012"
},
{
"DOI": "10.1002/jcph.1077",
"article-title": "Clinical pharmacokinetics of sulfobutylether-β-cyclodextrin in patients with varying degrees of renal impairment",
"author": "Hoover",
"doi-asserted-by": "crossref",
"first-page": "814",
"journal-title": "J Clin Pharmacol",
"key": "2024072210104968200_ciae333-B21",
"volume": "58",
"year": "2018"
},
{
"DOI": "10.1080/14740338.2021.1978976",
"article-title": "Comment about the safety of intravenous voriconazole formulated with sulfobutylether beta-cyclodextrin",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Exp Opin Drug Saf",
"key": "2024072210104968200_ciae333-B22",
"volume": "21",
"year": "2022"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae333/7697980"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and Safety of Remdesivir in People With Impaired Kidney Function Hospitalized for Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial",
"type": "journal-article"
}