Exploring the influence of vitamin C concentrations on the dynamics of RT-PCR assay reactions
et al., Scientific Reports, doi:10.1038/s41598-025-91154-1, Jul 2025
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing that vitamin C may interfere with COVID-19 RT-PCR diagnostics by altering reaction kinetics. High vitamin C concentrations (50-100 mg/ml) significantly increased Ct values in samples with high viral load while decreasing values in samples with low viral load. The concentrations are supraphysiologic - 50-100 mg ml⁻¹ far exceeds serum levels achieved by oral or standard IV dosing, so the finding is most relevant to intranasal or direct-sample contamination scenarios. In clinical treatment studies to date, benefit is seen only for serious outcomes, with no significant effect seen for viral outcomes.
17 preclinical studies support the efficacy of vitamin C for COVID-19:
Vitamin C has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function15-17.
Vitamin C plays a key role in the immune system, supporting the production and function of leukocytes, or white blood cells, which defend against infection and disease, including the production of lymphocytes, which make antibodies, and enhancing phagocytosis, the process by which immune system cells ingest and destroy viruses and infected cells.
Vitamin C is an antioxidant, protecting cells from damage caused by free radicals.
Vitamin C inhibits SARS-CoV-2 3CLpro7,11, inhibits SARS-CoV-2 infection by reducing ACE2 levels in a dose-dependent manner12, and may limit COVID-19 induced cardiac damage by acting as an antioxidant and potentially reducing the reactive oxygen species (ROS) production induced by the spike protein that contributes to the activation of profibrotic pathways9.
Vitamin C reduces inflammation, oxidative stress, and NETosis, supporting immune function and vascular protection18.
Intracellular levels of vitamin C decline during COVID-19 hospitalization suggesting ongoing utilization and depletion of vitamin C19.
Threonic acid, a metabolite of vitamin C, is lower in mild and severe cases, consistent with increased need for and metabolization of vitamin C with moderate infection, but more limited ability to produce threonic acid in severe infection due to depletion or existing lower levels of vitamin C20.
Symptomatic COVID-19 is associated with a lower frequency of natural killer (NK) cells, and vitamin C has been shown to improve NK cell numbers and functioning21,22.
1.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
2.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
3.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
4.
Morales-Bayuelo et al., New findings on ligand series used as SARS-CoV-2 virus inhibitors within the frameworks of molecular docking, molecular quantum similarity and chemical reactivity indices, F1000Research, doi:10.12688/f1000research.123550.3.
5.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
6.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
7.
Malla et al., Vitamin C inhibits SARS coronavirus-2 main protease essential for viral replication, bioRxiv, doi:10.1101/2021.05.02.442358.
8.
Kumar et al., In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19, VirusDisease, doi:10.1007/s13337-020-00643-6.
9.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
10.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
11.
Đukić et al., Inhibition of SARS-CoV-2 Mpro with Vitamin C, L-Arginine and a Vitamin C/L-Arginine Combination, Frontiers in Bioscience-Landmark, doi:10.31083/j.fbl2801008.
12.
Zuo et al., Vitamin C promotes ACE2 degradation and protects against SARS‐CoV‐2 infection, EMBO reports, doi:10.15252/embr.202256374.
13.
Hajdrik et al., In Vitro Determination of Inhibitory Effects of Humic Substances Complexing Zn and Se on SARS-CoV-2 Virus Replication, Foods, doi:10.3390/foods11050694.
14.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
15.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
16.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
17.
EFSA, Scientific Opinion on the substantiation of health claims related to vitamin C and protection of DNA, proteins and lipids from oxidative damage (ID 129, 138, 143, 148), antioxidant function of lutein (ID 146), maintenance of vision (ID 141, 142), collagen formation (ID 130, 131, 136, 137, 149), function of the nervous system (ID 133), function of the immune system (ID 134), function of the immune system during and after extreme physical exercise (ID 144), non-haem iron absorption (ID 132, 147), energy-yielding metabolism (ID 135), and relief in case of irritation in the upper respiratory tract (ID 1714, 1715) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2009.1226.
18.
Xie et al., The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death, Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6.
19.
Boerenkamp et al., Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients, Nutrients, doi:10.3390/nu15163653.
20.
Albóniga et al., Differential abundance of lipids and metabolites related to SARS-CoV-2 infection and susceptibility, Scientific Reports, doi:10.1038/s41598-023-40999-5.
Sharafi et al., 29 Jul 2025, peer-reviewed, 6 authors.
Contact: psharafi@etu.edu.tr.
Exploring the influence of vitamin C concentrations on the dynamics of RT-PCR assay reactions
Scientific Reports, doi:10.1038/s41598-025-91154-1
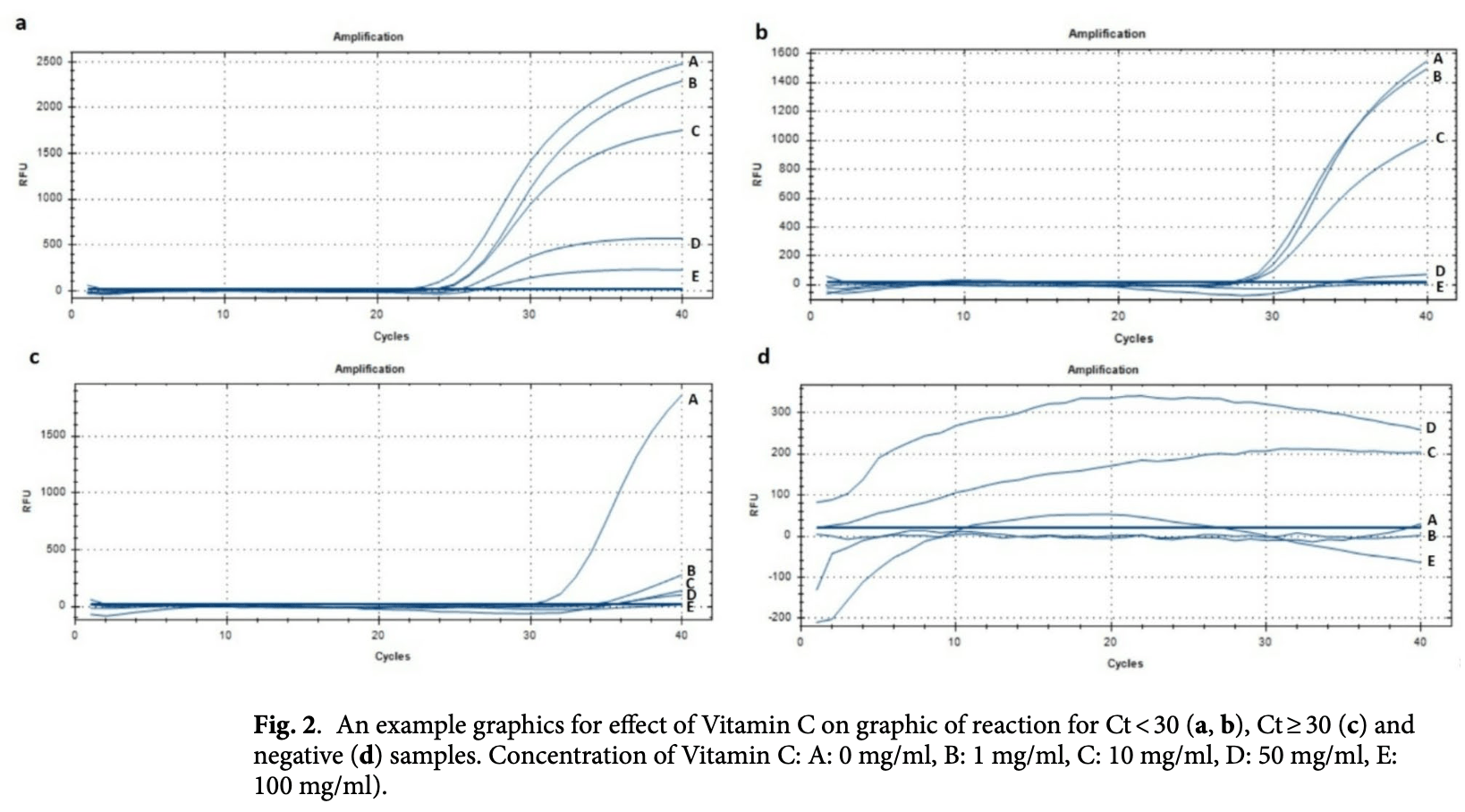
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused the COVID-19 pandemic to break out touched off a global health catastrophe characterized by various degrees of disease severity among those who were afflicted. Many strategies, such as vitamin C administration, have been investigated to reduce COVID-19 symptoms. Although the exact processes by which vitamin C affects COVID-19 remain unclear, noticeable changes in PCR test results were noted in our laboratory settings. This study uses PCR analysis to investigate the effects of varying vitamin C dosages and durations on COVID-19 test results. PCR cycle threshold (Ct) values were used to categorize nasopharyngeal tissues from 98 patients (Ct < 30, Ct ≥ 30, negative). Vitamin C was applied at different concentrations (0, 1, 10, 50, and 100 mg/ml), and PCR analyses were carried out at 1, 10, 24, and 48 h marks after the vitamin was applied. Particularly in samples with lower Ct values, the data showed significant changes in the reaction graphs and metrics with increasing vitamin C concentration. Higher concentrations of vitamin C were correlated with diminished metrics, occasionally leading to negative results for samples with Ct ≥ 30 values. Notably, samples that showed no discernible viral loads had different pictorial representations. These results raise questions regarding the reliability of PCR results in the presence of vitamin C intake and have implications for COVID-19 diagnosis. In light of the current pandemic, more studies are necessary to confirm and expand these findings and provide a critical understanding of clinical procedures and the interpretation of test results.
administered 0 and 1 mg/ml vitamin C were higher than those for samples administered 50 and 100 mg/ml vitamin C. Additionally, the median Ct values for 50 and 100 mg/ml vitamin C doses were very low, which was attributed to graphical image disruption in the PCR test. In the 10th hour for samples exhibiting a negative viral load, it was noted that at least one dose yielded distinct effects compared to others (χ 2 = 13.063; p = 0.011). Upon investigating the cause of this difference, a significant difference was observed between the 0 and 10 mg/ml doses (Z = 3.213; p = 0.013), whereas the other vitamin C doses did not exhibit statistical significance (p > 0.05). The median Ct value of samples administered 0 mg/ml vitamin C was higher than that of samples administered 10 mg/ml vitamin C. The PCR test reaction was affected by the deterioration of the graphic images, which was the reason for this discrepancy. In the 24th hour, for samples with a high viral load (Ct < 30), it was observed that at least one dose had a distinct effect compared to the others (χ 2 = 64.160; p < 0.001). Upon investigating the reason for this difference, statistically significant differences emerged between low doses (0, 1, and 10 mg/ml) and high doses (50 and 100 mg/ml) of Vitamin C (P < 0.05), with no significant difference between low and high doses (P > 0.05). The median Ct values of samples treated with low doses of Vitamin C were notably lower than those of samples treated with high..
References
Adhikari, Intravenous vitamin C for patients hospitalized with COVID-19: Two harmonized randomized clinical trials, JAMA
Arevalo-Rodriguez, False-negative results of initial RT-PCR assays for COVID-19: A systematic review
Artik, In-vitro for Q-RT-PCR clinical evaluation of Oscardia Ledovir spray effectiveness on SARS-CoV-2 and its effective variants, Explor. Res. Hypothesis Med
Bae, Kim, Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19, Molecules
Canali, Vitamin C supplementation modulates gene expression in peripheral blood mononuclear cells specifically upon an inflammatory stimulus: A pilot study in healthy subjects, Genes Nutr
Carr, Maggini, Vitamin C and immune function, Nutrients
Carr, Rowe, The emerging role of vitamin C in the prevention and treatment of COVID-19, Nutrients
Dosedel, Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination, Nutrients
Fogleman, A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19, J. Am. Board. Fam. Med
Hemilä, De Man, Vitamin C and COVID-19, Front. Med
Hiedra, The use of IV vitamin C for patients with COVID-19: A case series, Expert Rev. Anti Infect. Ther
Holford, Vitamin C-An adjunctive therapy for respiratory infection, sepsis and COVID-19, Nutrients
Investigators, on behalf of the Canadian Critical Care Trials Group, and the REMAP-CAP Investigators
Khorasani, Chegini, Mirzaei, New insight into laboratory tests and imaging modalities for fast and accurate diagnosis of COVID-19: Alternative suggestions for routine RT-PCR and CT-A literature review, Can. Respir. J
Klussmann, Early intervention with azelastine nasal spray May reduce viral load in SARS-CoV-2 infected patients, Sci. Rep
Kucirka, Lauer, Laeyendecker, Boon, Lessler, Variation in false-negative rate of reverse transcriptase polymerase chain Reaction-Based SARS-CoV-2 tests by time since exposure, Ann. Intern. Med
Kumar, Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial, J. Family Med. Prim. Care
Li, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, N Engl. J. Med
Li, Structure, function, and evolution of coronavirus spike proteins, Annu. Rev. Virol
Li, Use of intravenous vitamin C in critically ill patients with COVID-19 infection, J. Pharm. Pract
Moore, Khanna, The role of vitamin C in human immunity and its treatment potential against COVID-19: A review article, Cureus
Olczak-Pruc, Vitamin C supplementation for the treatment of COVID-19: A systematic review and meta-analysis, Nutrients
Padayatty, Levine, Vitamin, The known and the unknown and goldilocks, Oral Dis
Pecoraro, Negro, Pirotti, Trenti, Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis, Eur. J. Clin. Invest
Shamberger, Genetic toxicology of ascorbic acid, Mutat. Res
Tomasa-Irriguible, Bielsa-Berrocal, COVID-19: Up to 82% critically ill patients had low vitamin C values, Nutr. J
Weiss, Leibowitz, Coronavirus pathogenesis, Adv. Virus Res
Wikramaratna, Paton, Ghafari, Lourenço, Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR, Euro. Surveill
Yamasaki, Imai, Tanaka, Otaki, Pleiotropic functions of nitric oxide produced by ascorbate for the prevention and mitigation of COVID-19: A revaluation of Pauling's vitamin C therapy, Microorganisms
Yang, Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis, Biochem. Genet
Yuce, Filiztekin, Ozkaya, COVID-19 diagnosis-A review of current methods, Biosens. Bioelectron
Zhu, A novel coronavirus from patients with pneumonia in China, N Engl. J. Med
DOI record:
{
"DOI": "10.1038/s41598-025-91154-1",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-91154-1",
"alternative-id": [
"91154"
],
"article-number": "27545",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2 May 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 February 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "29 July 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All experiments involving human participants and the use of human tissue samples were performed in accordance with relevant guidelines and regulations. The study was approved by the Institutional Ethics Committee of TOBB University of Technology and Economy, ANKARA, and all participants provided written informed consent prior to their inclusion in the study. Detailed information regarding the approval can be found in the “Methods” section under the subheading “Ethical Approval”. The approval reference number is KAEK-118/144, and the date of approval was 11.11.2022. All methods were carried out in accordance with relevant guidelines and regulations."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors give the publisher the permission to publish the work."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-7400-4851",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sharafi",
"given": "Parisa",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0003-1449-7874",
"affiliation": [],
"authenticated-orcid": false,
"family": "Akyol",
"given": "Mesut",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8282-1360",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gultekin",
"given": "Ebrar",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0000-3908-7463",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sakar",
"given": "Rohat",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4109-0220",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ardicoglu Akisin",
"given": "N. Yasemin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8207-8749",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gocmen",
"given": "J. Sedef",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T05:16:02Z",
"timestamp": 1753766162000
},
"deposited": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T05:16:09Z",
"timestamp": 1753766169000
},
"indexed": {
"date-parts": [
[
2025,
8,
2
]
],
"date-time": "2025-08-02T19:07:44Z",
"timestamp": 1754161664267,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
7,
29
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T00:00:00Z",
"timestamp": 1753747200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T00:00:00Z",
"timestamp": 1753747200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-91154-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-91154-1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-91154-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
7,
29
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"author": "N Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl. J. Med.",
"key": "91154_CR1",
"unstructured": "Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl. J. Med. 382(8), 727–733 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001316",
"author": "Q Li",
"doi-asserted-by": "crossref",
"first-page": "1199",
"issue": "13",
"journal-title": "N Engl. J. Med.",
"key": "91154_CR2",
"unstructured": "Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl. J. Med. 382(13), 1199–1207 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.bios.2020.112752",
"author": "M Yuce",
"doi-asserted-by": "crossref",
"first-page": "112752",
"journal-title": "Biosens. Bioelectron.",
"key": "91154_CR3",
"unstructured": "Yuce, M., Filiztekin, E. & Ozkaya, K. G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 172, 112752 (2021).",
"volume": "172",
"year": "2021"
},
{
"author": "A Khorasani",
"first-page": "4648307",
"journal-title": "Can. Respir. J.",
"key": "91154_CR4",
"unstructured": "Khorasani, A., Chegini, A. & Mirzaei, A. New insight into laboratory tests and imaging modalities for fast and accurate diagnosis of COVID-19: Alternative suggestions for routine RT-PCR and CT-A literature review. Can. Respir. J. 2020, 4648307 (2020).",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/B978-0-12-385885-6.00009-2",
"author": "SR Weiss",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Adv. Virus Res.",
"key": "91154_CR5",
"unstructured": "Weiss, S. R. & Leibowitz, J. L. Coronavirus pathogenesis. Adv. Virus Res. 81, 85–164 (2011).",
"volume": "81",
"year": "2011"
},
{
"DOI": "10.1146/annurev-virology-110615-042301",
"author": "F Li",
"doi-asserted-by": "crossref",
"first-page": "237",
"issue": "1",
"journal-title": "Annu. Rev. Virol.",
"key": "91154_CR6",
"unstructured": "Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 3(1), 237–261 (2016).",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1007/s10528-013-9574-0",
"author": "H Yang",
"doi-asserted-by": "crossref",
"first-page": "413",
"issue": "5–6",
"journal-title": "Biochem. Genet.",
"key": "91154_CR7",
"unstructured": "Yang, H. Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem. Genet. 51(5–6), 413–425 (2013).",
"volume": "51",
"year": "2013"
},
{
"DOI": "10.1111/odi.12446",
"author": "SJ Padayatty",
"doi-asserted-by": "crossref",
"first-page": "463",
"issue": "6",
"journal-title": "Oral Dis.",
"key": "91154_CR8",
"unstructured": "Padayatty, S. J., Levine, M. & Vitamin, C. The known and the unknown and goldilocks. Oral Dis. 22(6), 463–493 (2016).",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1007/s12263-014-0390-x",
"author": "R Canali",
"doi-asserted-by": "crossref",
"first-page": "390",
"issue": "3",
"journal-title": "Genes Nutr.",
"key": "91154_CR9",
"unstructured": "Canali, R. et al. Vitamin C supplementation modulates gene expression in peripheral blood mononuclear cells specifically upon an inflammatory stimulus: A pilot study in healthy subjects. Genes Nutr. 9(3), 390 (2014).",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.3390/nu13020615",
"doi-asserted-by": "crossref",
"key": "91154_CR10",
"unstructured": "Dosedel, M. et al. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 13(2) (2021)."
},
{
"DOI": "10.3390/molecules25225346",
"doi-asserted-by": "crossref",
"key": "91154_CR11",
"unstructured": "Bae, M. & Kim, H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 25(22) (2020)."
},
{
"DOI": "10.3390/nu9111211",
"doi-asserted-by": "crossref",
"key": "91154_CR12",
"unstructured": "Carr, A. C. & Maggini, S. Vitamin C and immune function. Nutrients 9(11) (2017)."
},
{
"DOI": "10.4103/jfmpc.jfmpc_2437_21",
"author": "V Kumar",
"doi-asserted-by": "crossref",
"first-page": "4758",
"issue": "8",
"journal-title": "J. Family Med. Prim. Care",
"key": "91154_CR13",
"unstructured": "Kumar, V. et al. Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial. J. Family Med. Prim. Care 11(8), 4758–4765 (2022).",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3390/nu14194217",
"author": "M Olczak-Pruc",
"doi-asserted-by": "crossref",
"first-page": "4217",
"issue": "19",
"journal-title": "Nutrients",
"key": "91154_CR14",
"unstructured": "Olczak-Pruc, M. et al. Vitamin C supplementation for the treatment of COVID-19: A systematic review and meta-analysis. Nutrients 14(19), 4217 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2020.559811",
"author": "H Hemilä",
"doi-asserted-by": "crossref",
"first-page": "559811",
"journal-title": "Front. Med.",
"key": "91154_CR15",
"unstructured": "Hemilä, H. & de Man, A. M. E. Vitamin C and COVID-19. Front. Med. 7, 559811 (2021).",
"volume": "7",
"year": "2021"
},
{
"author": "A Moore",
"first-page": "e33740",
"issue": "1",
"journal-title": "Cureus",
"key": "91154_CR16",
"unstructured": "Moore, A. & Khanna, D. The role of vitamin C in human immunity and its treatment potential against COVID-19: A review article. Cureus 15(1), e33740 (2023).",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/nu12113286",
"author": "AC Carr",
"doi-asserted-by": "crossref",
"first-page": "3286",
"issue": "11",
"journal-title": "Nutrients",
"key": "91154_CR17",
"unstructured": "Carr, A. C. & Rowe, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 12(11), 3286 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/s12937-021-00727-z",
"author": "TM Tomasa-Irriguible",
"doi-asserted-by": "crossref",
"first-page": "66",
"issue": "1",
"journal-title": "Nutr. J.",
"key": "91154_CR18",
"unstructured": "Tomasa-Irriguible, T. M. & Bielsa-Berrocal, L. COVID-19: Up to 82% critically ill patients had low vitamin C values. Nutr. J. 20(1), 66 (2021).",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.3390/nu12123760",
"author": "P Holford",
"doi-asserted-by": "crossref",
"first-page": "3760",
"issue": "12",
"journal-title": "Nutrients",
"key": "91154_CR19",
"unstructured": "Holford, P. et al. Vitamin C—An adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients 12(12), 3760 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2020.1794819",
"author": "R Hiedra",
"doi-asserted-by": "crossref",
"first-page": "1259",
"issue": "12",
"journal-title": "Expert Rev. Anti Infect. Ther.",
"key": "91154_CR20",
"unstructured": "Hiedra, R. et al. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Infect. Ther. 18(12), 1259–1261 (2020).",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1177/08971900211015052",
"author": "M Li",
"doi-asserted-by": "crossref",
"first-page": "60",
"issue": "1",
"journal-title": "J. Pharm. Pract.",
"key": "91154_CR21",
"unstructured": "Li, M. et al. Use of intravenous vitamin C in critically ill patients with COVID-19 infection. J. Pharm. Pract. 36(1), 60–66 (2023).",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.3122/jabfm.2022.04.210529",
"doi-asserted-by": "crossref",
"key": "91154_CR22",
"unstructured": "Fogleman, C. et al. A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19. J. Am. Board. Fam. Med. 35(4), 695–707 (2022)."
},
{
"DOI": "10.1001/jama.2023.21407",
"doi-asserted-by": "crossref",
"key": "91154_CR23",
"unstructured": "LOVIT-COVID Investigators, on behalf of the Canadian Critical Care Trials Group, and the REMAP-CAP Investigators; Adhikari, N. K. J. et al. Intravenous vitamin C for patients hospitalized with COVID-19: Two harmonized randomized clinical trials. JAMA 330(18), 1745–1759 (2023)."
},
{
"DOI": "10.1111/eci.13706",
"author": "V Pecoraro",
"doi-asserted-by": "crossref",
"first-page": "e13706",
"issue": "2",
"journal-title": "Eur. J. Clin. Invest.",
"key": "91154_CR24",
"unstructured": "Pecoraro, V., Negro, A., Pirotti, T. & Trenti, T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur. J. Clin. Invest. 52(2), e13706 (2022).",
"volume": "52",
"year": "2022"
},
{
"key": "91154_CR25",
"unstructured": "Arevalo-Rodriguez, I. et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review."
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.50.2000568",
"author": "PS Wikramaratna",
"doi-asserted-by": "crossref",
"first-page": "2000568",
"issue": "50",
"journal-title": "Euro. Surveill",
"key": "91154_CR26",
"unstructured": "Wikramaratna, P. S., Paton, R. S., Ghafari, M. & Lourenço, J. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Euro. Surveill. 25(50), 2000568 (2020).",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/0165-1110(84)90005-8",
"author": "RJ Shamberger",
"doi-asserted-by": "crossref",
"first-page": "135",
"issue": "2",
"journal-title": "Mutat. Res.",
"key": "91154_CR27",
"unstructured": "Shamberger, R. J. Genetic toxicology of ascorbic acid. Mutat. Res. 133(2), 135–159 (1984).",
"volume": "133",
"year": "1984"
},
{
"DOI": "10.1038/s41598-023-32546-z",
"author": "JP Klussmann",
"doi-asserted-by": "crossref",
"first-page": "6839",
"journal-title": "Sci. Rep.",
"key": "91154_CR28",
"unstructured": "Klussmann, J. P. et al. Early intervention with azelastine nasal spray May reduce viral load in SARS-CoV-2 infected patients. Sci. Rep. 13, 6839 (2023).",
"volume": "13",
"year": "2023"
},
{
"author": "Y Artik",
"first-page": "119",
"issue": "2",
"journal-title": "Explor. Res. Hypothesis Med.",
"key": "91154_CR29",
"unstructured": "Artik, Y. et al. In-vitro for Q-RT-PCR clinical evaluation of Oscardia Ledovir spray effectiveness on SARS-CoV-2 and its effective variants. Explor. Res. Hypothesis Med. 8(2), 119–132 (2023).",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3390/microorganisms11020397",
"author": "H Yamasaki",
"doi-asserted-by": "crossref",
"first-page": "397",
"issue": "2",
"journal-title": "Microorganisms",
"key": "91154_CR30",
"unstructured": "Yamasaki, H., Imai, H., Tanaka, A. & Otaki, J. M. Pleiotropic functions of nitric oxide produced by ascorbate for the prevention and mitigation of COVID-19: A revaluation of Pauling’s vitamin C therapy. Microorganisms 11(2), 397 (2023).",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.7326/M20-1495",
"author": "LM Kucirka",
"doi-asserted-by": "crossref",
"first-page": "262",
"issue": "4",
"journal-title": "Ann. Intern. Med.",
"key": "91154_CR31",
"unstructured": "Kucirka, L. M., Lauer, S. A., Laeyendecker, O., Boon, D. & Lessler, J. Variation in false-negative rate of reverse transcriptase polymerase chain Reaction-Based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 173(4), 262–267 (2020).",
"volume": "173",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-91154-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Exploring the influence of vitamin C concentrations on the dynamics of RT-PCR assay reactions",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
