Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2022.2098059, NCT04707742, Jul 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
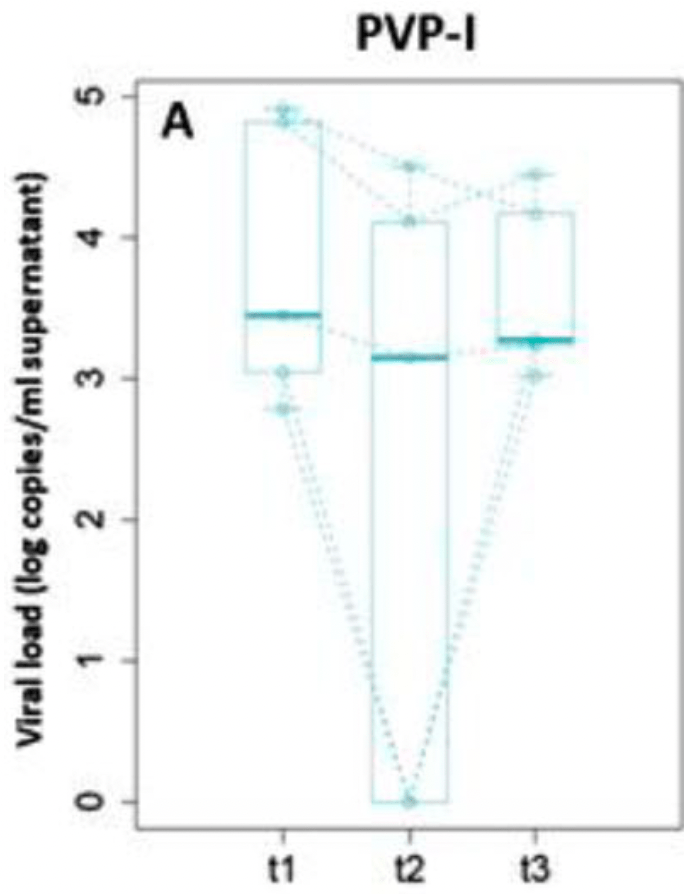
RCT hospitalized patients testing viral load shortly after a single mouthwash with PVP-I, hydrogen peroxide, cetylpyridinium chloride, chlorhexidine, and water. For PVP-I, there were only 5 patients with viable virus at baseline. Cross-treatment comparisons are limited due to the very small sample sizes and large difference between baseline viral loads (the baseline viral culture non-log copies/mL was ~10,000 times higher in the water group vs. PVP-I).
The trial registration shows that 160 patients were recruited, with 62 patients added in an October 6, 2021 change, however the paper presents results for only 75 patients.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Study covers cetylpyridinium chloride, hydrogen peroxide, and povidone-iodine.
|
risk of viral load, 33.9% lower, RR 0.66, treatment 5, control 5, relative viral culture non-log median copies/mL, 60 minutes vs. baseline.
|
|
risk of viral load, 49.9% lower, RR 0.50, treatment 5, control 5, relative viral culture non-log median copies/mL, 30 minutes vs. baseline.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sánchez Barrueco et al., 27 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, mean age 55.0, 17 authors, study period 15 June, 2020 - 30 September, 2021, trial NCT04707742 (history).
Contact: mira_ale@gva.es.
Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial
Emerging Microbes & Infections, doi:10.1080/22221751.2022.2098059
Background: In vitro studies have shown that several oral antiseptics have virucidal activity against SARS-CoV-2. Thus, mouthwashes have been proposed as an easy to implement strategy to reduce viral transmission. However, there are no data measuring SARS-CoV-2 viability after mouthwashes in vivo. Methods: In this randomized double-blind, fiveparallel-group, placebo-controlled clinical trial, SARS-CoV-2 salivary viral load (by quantitative PCR) and its infectious capacity (incubating saliva in cell cultures) have been evaluated before and after four different antiseptic mouthwashes and placebo in 54 COVID-19 patients. Results: Contrary to in vitro evidence, salivary viral load was not affected by any of the four tested mouthwashes. Viral culture indicated that cetylpyridinium chloride (CPC) significantly reduced viral infectivity, but only at 1-hour post-mouthwash. Conclusion: These results indicate that some of the mouthwashes currently used to reduce viral infectivity are not efficient in vivo and, furthermore, that this effect is not immediate, generating a false sense of security.
Disclosure statement No potential conflict of interest was reported by the author(s).
Author contributions
References
Ausina-Marquez, Oral antiseptics against coronavirus: in-vitro and clinical evidence, J Hosp Infect
Biber, Lev, Mandelboim, The role of mouthwash sampling in SARS-CoV-2 diagnosis, Eur J Clin Microbiol Infect Dis
Cdc, Guidance for dental settings: interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet
Core, R: A language and environment for statistical computing
Costa, Brites, Vaz, Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: A randomized clinical trial, Oral Dis, doi:10.1111/odi.14086
Davies, Buczkowski, Welch, Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes, J Gen Virol
Deana, Seiffert, Aravena-Rivas, Recommendations for safe dental care: A Systematic review of clinical practice guidelines in the first year of the COVID-19 pandemic, Int J Environ Res Public Health
Eduardo, Corrêa, Heller, Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial, Heliyon
Elzein, Sater, Fakhreddine, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract
Ferrer, Barrueco, Martinez-Beneyto, Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Sci Rep
Gottsauner, Michaelides, Schmidt, A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin Oral Investig
Huang, Huang, Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients, J Med Virol
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology
Koch-Heier, Hoffmann, Schindler, Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX® and BacterX® Pro, Microorganisms
Komine, Yamaguchi, Okamoto, Virucidal activity of oral care products against SARS-CoV-2 in vitro, J Oral Maxillofac Surg Med Pathol
Kowalski, Sanabria, Ridge, COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice, Head Neck
Lamas, Dios, Rodríguez, Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis
Mendoza, Ubillus, Bolivar, Antiviral effect of mouthwashes against SARS-COV-2: A systematic review, Saudi Dent J
Meyers, Robison, Milici, Lowering the transmission and spread of human coronavirus, J Med Virol
Munoz-Basagoiti, Perez-Zsolt, Leon, Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, J Dent Res
Pelletier, Tessema, Frank, Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), Ear Nose Throat J
Peng, Xu, Li, Transmission routes of 2019-nCoV and controls in dental practice, Int J Oral Sci
Schurmann, Aljubeh, Tiemann, Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study, Eur Arch Otorhinolaryngol
Schutz, Conzelmann, Fois, Carrageenancontaining over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures, Am J Physiol Lung Cell Mol Physiol
Scola, Bideau, Andreani, Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards, Eur J Clin Microbiol Infect Dis
Seneviratne, Balan, Ko, Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Sreebny, Saliva in health and disease: an appraisal and update, Int Dent J
Steinhauer, Meister, Todt, Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European standard EN 14476, J Hosp Infect
Tadakamadla, Boccalari, Rathore, In vitro studies evaluating the efficacy of mouth rinses on sars-Cov-2: A systematic review, J Infect Public Health
To, Tsang, Leung, Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis
To, Tsang, Yip, Consistent detection of 2019 novel coronavirus in saliva, Clin Infect Dis
Wang, Wu, Wang, Virucidal effect of povidone-iodine against SARS-CoV-2 in vitro, J Int Med Res
Whitworth, COVID-19: a fast evolving pandemic, Trans R Soc Trop Med Hyg
Worldometer, Coronavirus Cases
Xu, Wang, Hoskin, Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro, Pathogens
Yoon, Yoon, Song, Clinical significance of a high SARS-CoV-2 viral load in the saliva, J Korean Med Sci
DOI record:
{
"DOI": "10.1080/22221751.2022.2098059",
"ISSN": [
"2222-1751"
],
"URL": "http://dx.doi.org/10.1080/22221751.2022.2098059",
"alternative-id": [
"10.1080/22221751.2022.2098059"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-05"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2022-06-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-06-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-07-27"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7919-5765",
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
},
{
"name": "ENT and Cervicofacial Surgery Department, Villalba General University Hospital, Collado Villalba, Spain"
}
],
"authenticated-orcid": false,
"family": "Sánchez Barrueco",
"given": "Álvaro",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8237-4596",
"affiliation": [
{
"name": "Department of Dental Clinical Specialties, School of Dentistry, Madrid Complutense University, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Mateos-Moreno",
"given": "María Victoria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1523-9415",
"affiliation": [
{
"name": "Department of Dermatology, Stomatology and Radiology, University of Murcia, Murcia, Spain"
},
{
"name": "Murcian Institute of Biosanitary Research (IMIB), Murcia, Spain"
}
],
"authenticated-orcid": false,
"family": "Martínez-Beneyto",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Virgen de la Arrixaca University Clinical Hospital, IMIB, Murcia, Spain"
}
],
"family": "García-Vázquez",
"given": "Elisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7742-7497",
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Campos González",
"given": "Alfonso",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
},
{
"name": "Villalba General University Hospital, Collado Villalba, Spain"
}
],
"family": "Zapardiel Ferrero",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
}
],
"family": "Bogoya Castaño",
"given": "Abel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3309-187X",
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Villalba General University Hospital, Collado Villalba, Spain"
}
],
"authenticated-orcid": false,
"family": "Alcalá Rueda",
"given": "Ignacio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1321-007X",
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Villacampa Aubá",
"given": "José Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ENT and Cervicofacial Surgery Department, Fundación Jiménez Díaz University Hospital, Madrid, Spain"
}
],
"family": "Cenjor Español",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Service, Murcian Institute of Biosanitary Research, Virgen de la Arrixaca University Clinical Hospital, Murcia, Spain"
}
],
"family": "Moreno-Parrado",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Dentistry, European University of Valencia, Valencia, Spain"
}
],
"family": "Ausina-Márquez",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Genomics & Health Department, FISABIO-Public Health Foundation, Valencia, Spain"
}
],
"family": "García-Esteban",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Genomics & Health Department, FISABIO-Public Health Foundation, Valencia, Spain"
}
],
"family": "Artacho",
"given": "Alejandro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9403-8258",
"affiliation": [
{
"name": "Genomics & Health Department, FISABIO-Public Health Foundation, Valencia, Spain"
},
{
"name": "Department of Microbiology and Ecology, Medical School, University of Valencia, Valencia, Spain"
},
{
"name": "CIBER in Epidemiology and Public Health (CIBERESP), Instituto de Salud Carlos III, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "López-Labrador",
"given": "F. Xavier",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9127-3877",
"affiliation": [
{
"name": "Genomics & Health Department, FISABIO-Public Health Foundation, Valencia, Spain"
},
{
"name": "CIBER in Epidemiology and Public Health (CIBERESP), Instituto de Salud Carlos III, Madrid, Spain"
}
],
"authenticated-orcid": false,
"family": "Mira",
"given": "Alex",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4521-1632",
"affiliation": [
{
"name": "Genomics & Health Department, FISABIO-Public Health Foundation, Valencia, Spain"
}
],
"authenticated-orcid": false,
"family": "Ferrer",
"given": "María D.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04707742",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Emerging Microbes & Infections",
"container-title-short": "Emerging Microbes & Infections",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
7
]
],
"date-time": "2022-07-07T08:15:23Z",
"timestamp": 1657181723000
},
"deposited": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T19:18:46Z",
"timestamp": 1659381526000
},
"funder": [
{
"DOI": "10.13039/501100011597",
"doi-asserted-by": "publisher",
"name": "Consellería de Sanitat Universal i Salut Pública"
},
{
"name": "European Fund of Regional Development / European Social Fund"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T19:41:39Z",
"timestamp": 1659382899411
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
7,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
27
]
],
"date-time": "2022-07-27T00:00:00Z",
"timestamp": 1658880000000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/22221751.2022.2098059",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1833-1842",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2022,
7,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "CIT0001",
"unstructured": "Worldometer. Coronavirus Cases. 2022; [cited May 27 2022]. Available from: https://www.worldometers.info/coronavirus/."
},
{
"DOI": "10.1093/trstmh/traa025",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"DOI": "10.1093/cid/ciaa149",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.1007/s10096-021-04320-4",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"key": "#cr-split#-CIT0006.1",
"unstructured": "Centers for Disease Control and Prevention - CDC. (2020). Guidance for dental settings: interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic"
},
{
"key": "#cr-split#-CIT0006.2",
"unstructured": "[Cited 2022 Jan 18]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html."
},
{
"DOI": "10.1016/j.jhin.2021.04.004",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.3390/pathogens10030272",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.1099/jgv.0.001578",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.1016/j.jiph.2021.07.020",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1016/j.ajoms.2021.02.002",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"DOI": "10.1177/03000605211063695",
"doi-asserted-by": "publisher",
"key": "CIT0014"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.1111/odi.13526",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.1007/s10096-020-03913-9",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1177/0145561320957237",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"DOI": "10.3390/ijerph181910059",
"doi-asserted-by": "publisher",
"key": "CIT0023"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1002/jmv.26514",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"DOI": "10.1016/j.jhin.2021.01.031",
"doi-asserted-by": "publisher",
"key": "CIT0026"
},
{
"DOI": "10.1111/j.1875-595X.2000.tb00554.x",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"DOI": "10.1007/s00405-021-06873-8",
"doi-asserted-by": "publisher",
"key": "CIT0028"
},
{
"DOI": "10.1002/jmv.26954",
"doi-asserted-by": "publisher",
"key": "CIT0029"
},
{
"DOI": "10.1111/odi.14086",
"doi-asserted-by": "publisher",
"key": "CIT0030"
},
{
"DOI": "10.1016/j.heliyon.2021.e07346",
"doi-asserted-by": "publisher",
"key": "CIT0031"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"doi-asserted-by": "publisher",
"key": "CIT0032"
},
{
"DOI": "10.1038/s41368-020-0075-9",
"doi-asserted-by": "publisher",
"key": "CIT0033"
},
{
"DOI": "10.1002/hed.26164",
"doi-asserted-by": "publisher",
"key": "CIT0034"
},
{
"DOI": "10.1016/j.sdentj.2022.01.006",
"doi-asserted-by": "publisher",
"key": "CIT0035"
},
{
"DOI": "10.1152/ajplung.00552.2020",
"doi-asserted-by": "publisher",
"key": "CIT0036"
},
{
"author": "R Core Team",
"key": "CIT0037",
"volume-title": "R: A language and environment for statistical computing",
"year": "2020"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/22221751.2022.2098059"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Drug Discovery",
"General Medicine",
"Immunology",
"Microbiology",
"Parasitology",
"Epidemiology"
],
"subtitle": [],
"title": "Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "11"
}
