Optimal dose and safety of intravenous favipiravir in hospitalised patients with SARS-CoV-2 infection: a Phase Ib, open-label, dose-escalating, randomised controlled study
et al., medRxiv, doi:10.1101/2025.06.09.25329141, AGILE, NCT04746183, Jun 2025
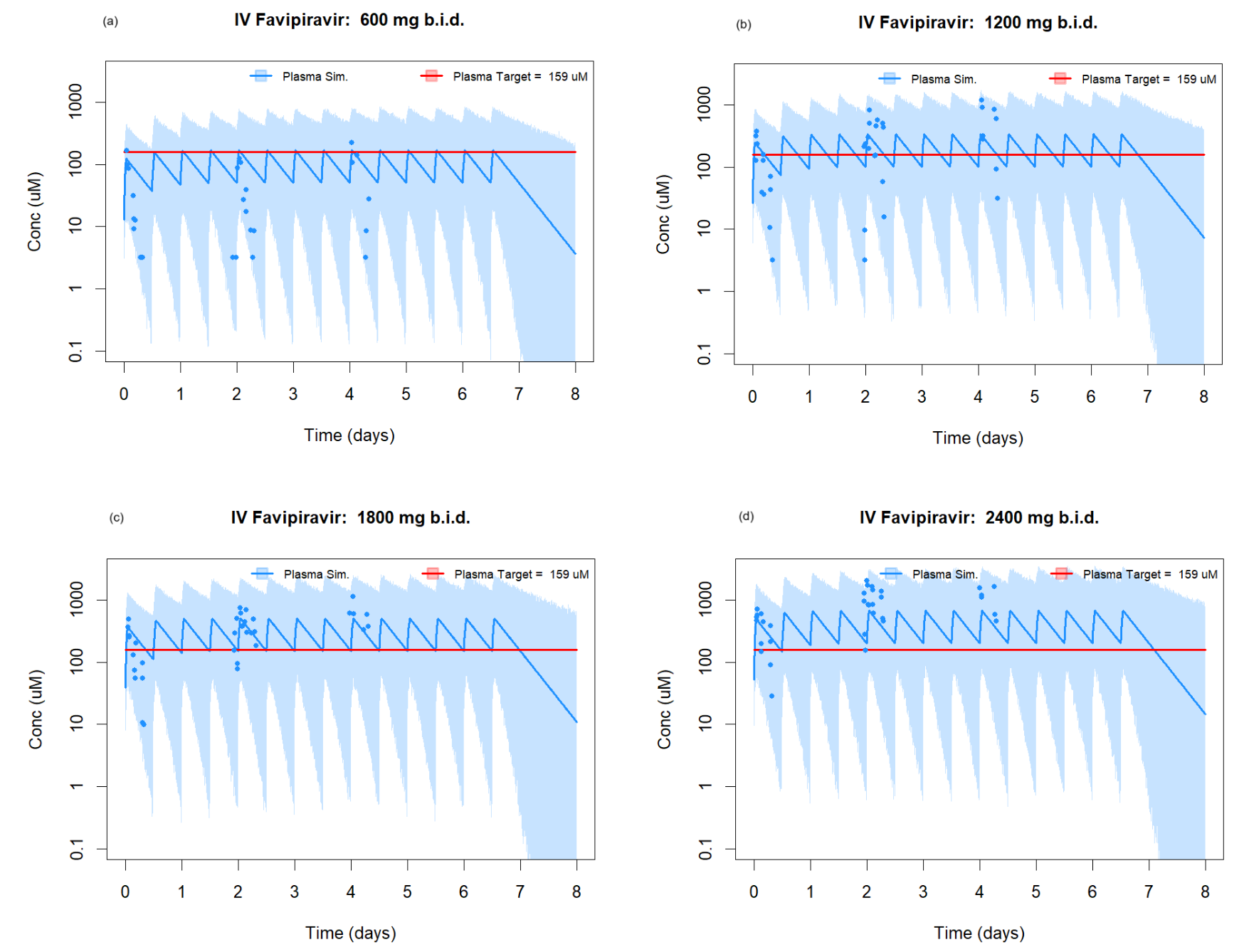
RCT 24 hospitalized COVID-19 patients (16 treatment, 8 standard of care) evaluating safety and pharmacokinetics of intravenous favipiravir at escalating doses. The study found that IV favipiravir was safe and well-tolerated up to 2400mg twice daily, with no dose-limiting toxicities observed. Significant inter-individual variability in pharmacokinetic parameters was observed. The 2400mg twice daily dose reached pre-specified target plasma concentrations throughout dosing. Authors conclude that IV favipiravir at these doses could be considered for future efficacy studies.
Authors report that "there was no difference in the median WHO score between the treatment and SoC groups at baseline, day 8, day 15 and day 29. The 2400mg b.i.d. cohort had lower median WHO scores than the SoC group at days 8 and 15", however the actual results are not provided.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Rowland et al., 9 Jun 2025, Randomized Controlled Trial, United Kingdom, preprint, median age 74.0, 32 authors, study period 10 September, 2022 - 1 November, 2023, trial NCT04746183 (history) (AGILE).
Optimal dose and safety of intravenous favipiravir in hospitalised patients with SARS-CoV-2 infection: a Phase Ib, open-label, dose-escalating, randomised controlled study
doi:10.1101/2025.06.09.25329141
Key points • A novel intravenous formulation of favipiravir, was safe and well tolerated in a frail and complex population, up to a dose of 2400mg b.i.d. • Significant inter-individual variability in pharmacokinetic parameters was observed. • Pharmacokinetic modelling suggests pre-specified target concentrations were met. .
References
Abdelnabi, Foo, Kaptein, The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Arshad, Pertinez, Box, Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther
Bixler, Bocan, Wells, Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus, Antiviral Res
Challenger, Penchala, Hale, Development and validation of an LC-MS/MS method for quantification of favipiravir in human plasma, J Pharm Biomed Anal
Choi, Shin, Park, Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes, Antiviral Res
Choy, Wong, Kaewpreedee, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res
Driouich, Cochin, Lingas, Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun
Du, Chen, Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection, Clin Pharmacol Ther, doi:10.1002/cpt.1844
Eloy, Solas, Touret, Dose Rationale for Favipiravir Use in Patients Infected With SARS-CoV-2, Clin Pharmacol Ther, doi:10.1002/cpt.1877
Fidler, Wilkins, Hooijmaijers, Nonlinear Mixed-Effects Model Development and Simulation Using nlmixr and Related R Open-Source Packages, CPT Pharmacometrics Syst Pharmacol
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Griffiths, Fitzgerald, Jaki, AGILE: a seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment: an update to the structured summary of a study protocol for a randomised platform trial letter, Trials, doi:10.1186/s13063-021-05458-4
Gülhan, Eryüksel, İdriz Oğlu, Pharmacokinetic characterization of favipiravir in patients with COVID-19, Br J Clin Pharmacol
Hattori, Higshi-Kuwata, Raghavaiah, GRL-0920, an Indole Chloropyridinyl Ester, Completely Blocks SARS-CoV-2 Infection, mBio
Hayden, Lenk, Epstein, Kang, Oral Favipiravir Exposure and Pharmacodynamic Effects in Outpatient Adults with Acute Influenza, J Infect Dis
Hayden, Lenk, Stonis, Oldham-Creamer, Kang et al., Favipiravir Treatment of Uncomplicated Influenza in Adults: Results of Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Trials, J Infect Dis
Irie, Nakagawa, Fujita, Pharmacokinetics of Favipiravir in Critically Ill Patients With COVID-19, Clin Transl Sci
Irie, Nakagawa, Fujita, Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol
Jaki, Barnett, Titman, Mozgunov, A seamless Phase I/II platform design with a time-to-event efficacy endpoint for potential COVID-19 therapies, Stat Methods Med Res
Jeon, Ko, Lee, Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, Antimicrob Agents Chemother
Kaptein, Jacobs, Langendries, Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci U S A
Khoo, Fitzgerald, Fletcher, Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study, J Antimicrob Chemother
Korula, Alexander, John, Favipiravir for treating COVID-19, Cochrane Database of Systematic Reviews
Madelain, Guedj, Mentre, Favipiravir Pharmacokinetics in Nonhuman Primates and Insights for Future Efficacy Studies of Hemorrhagic Fever Viruses, Antimicrob Agents Chemother
Mozgunov, Jaki, Paoletti, Randomized dose-escalation designs for drug combination cancer trials with immunotherapy, J Biopharm Stat
Nguyen, Guedj, Anglaret, Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted, PLoS Negl Trop Dis
Pertinez, Rajoli, Khoo, Owen, Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5′-triphosphate exposure to support posology for SARS-CoV-2, Journal of Antimicrobial Chemotherapy
Pizzorno, Padey, Dubois, In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2, Antiviral Res
Sakurai, Phase, Randomized, Double-blind, Placebo-controlled, Single Ascendingdose Study to Evaluate the Pharmacokinetics, Safety and Tolerability of Injectable Favipiravir in Healthy Subjects
Shannon, Selisko, Le, Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis, Nat Commun
Sissoko, Laouenan, Folkesson, Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea, PLoS Med
Walker, Fitzgerald, Saunders, An Open Label, Adaptive, Phase 1 Trial of High-Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS-CoV-2, Clin Pharmacol Ther
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Research
Wang, Zhong, Salam, Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza, EBioMedicine
DOI record:
{
"DOI": "10.1101/2025.06.09.25329141",
"URL": "http://dx.doi.org/10.1101/2025.06.09.25329141",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>AGILE (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04746183\">NCT04746183</jats:ext-link>) is a Phase Ib/IIa platform, evaluating candidates to treat COVID-19. CST-6 evaluated the safety and optimal dose of a novel intravenous (IV) formulation of favipiravir.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>CST-6 was a dose-escalating, open-label, randomised, controlled, Bayesian adaptive Phase Ib trial. Hospitalised adults with PCR-confirmed SARS-CoV-2 infection, within 14 days of symptomatic COVID-19 were randomised 2:1 in groups of 6 (n = 4 favipiravir, n = 2 standard of care (SoC)) to ascending doses of IV favipiravir twice daily (b.i.d.) for 7 days or SoC. Clinical data, safety evaluations, virology and pharmacokinetic (PK) samples were collected. The primary outcome was safety. Secondary outcomes included clinical, PK and virological endpoints.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>24 participants enrolled between 10/Sep/2022 and 01/Nov/2023 [10/24 female; median age 74 years (range 52-93)]. Favipiravir was well tolerated despite a high background rate of unrelated adverse events (AEs). No dose limiting toxicities (DLTs) were observed, with a model-predicted DLT risk of 16.8% and probability of unacceptable toxicity of 2.7% at the highest dose level. No SAEs were deemed related to favipiravir but an expected association with asymptomatic, transient hyperuricaemia was observed. PK exposures increased disproportionally to dose with significant accumulation in plasma, but with marked variability between participants within each cohort.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>A novel formulation of favipiravir was safe at sustained high doses that reached PK targets in a study group with frailty and complex health profiles. Plasma concentrations demonstrated accumulation. Significant variability in PK parameters between individuals was noted. We consider doses up to 2400mg b.i.d. to be safe for further evaluation.<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/study/NCT04746183\">https://clinicaltrials.gov/study/NCT04746183</jats:ext-link></jats:p></jats:sec><jats:sec><jats:title>Key points</jats:title><jats:list list-type=\"bullet\"><jats:list-item><jats:p>A novel intravenous formulation of favipiravir, was safe and well tolerated in a frail and complex population, up to a dose of 2400mg b.i.d.</jats:p></jats:list-item><jats:list-item><jats:p>Significant inter-individual variability in pharmacokinetic parameters was observed.</jats:p></jats:list-item><jats:list-item><jats:p>Pharmacokinetic modelling suggests pre-specified target concentrations were met.</jats:p></jats:list-item></jats:list></jats:sec>",
"accepted": {
"date-parts": [
[
2025,
6,
9
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0001-5059-065X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rowland",
"given": "Tim",
"sequence": "first"
},
{
"affiliation": [],
"family": "FitzGerald",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Challenger",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dickinson",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Else",
"given": "Laura J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hale",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaw",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kelly",
"given": "Callum",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyon",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibney",
"given": "Jenn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhamani",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Irwin",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Enever",
"given": "Yvanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tetlow",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wood",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reynolds",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiong",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osanlou",
"given": "Orod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pertinez",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bullock",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenhalf",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lalloo",
"given": "David G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiscox",
"given": "Julian A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mozgunov",
"given": "Pavel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Geoff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khoo",
"given": "Saye H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fletcher",
"given": "Tom E.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
6,
9
]
],
"date-time": "2025-06-09T20:40:18Z",
"timestamp": 1749501618000
},
"deposited": {
"date-parts": [
[
2025,
6,
11
]
],
"date-time": "2025-06-11T15:25:24Z",
"timestamp": 1749655524000
},
"group-title": "Pharmacology and Therapeutics",
"indexed": {
"date-parts": [
[
2025,
6,
12
]
],
"date-time": "2025-06-12T04:12:41Z",
"timestamp": 1749701561151,
"version": "3.41.0"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6,
9
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
9
]
],
"date-time": "2025-06-09T00:00:00Z",
"timestamp": 1749427200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.06.09.25329141",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
6,
9
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
6,
9
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1186/s13063-021-05352-z",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.1"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.2"
},
{
"DOI": "10.1016/j.ebiom.2020.103125",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.3",
"unstructured": "Wang Y , Zhong W , Salam A , et al. Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. EBioMedicine 2020; 62. Available at: https://pubmed.ncbi.nlm.nih.gov/33232871/. Accessed 13 August 2024."
},
{
"key": "2025061108251181000_2025.06.09.25329141v1.4",
"unstructured": "Hayden FG , Lenk RP , Epstein C , Kang LL . Oral Favipiravir Exposure and Pharmacodynamic Effects in Outpatient Adults with Acute Influenza. J Infect Dis 2023; Available at: http://www.ncbi.nlm.nih.gov/pubmed/37739792. Accessed 12 August 2024."
},
{
"DOI": "10.1371/journal.pmed.1002066",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.5",
"unstructured": "Sissoko D , Laouenan C , Folkesson E , et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med 2016; 13. Available at: https://pubmed.ncbi.nlm.nih.gov/26930627/. Accessed 15 August 2024."
},
{
"DOI": "10.1002/14651858.CD015219.pub2",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.6",
"unstructured": "Korula P , Alexander H , John JS , et al. Favipiravir for treating COVID-19. Cochrane Database of Systematic Reviews 2024; 2024."
},
{
"key": "2025061108251181000_2025.06.09.25329141v1.7",
"unstructured": "Evaluation and Licensing Division P and FSB, Ministry of Health L and W. Report on the Deliberation Results. Available at: https://www.pmda.go.jp/files/000210319.pdf. Accessed 27 August 2024."
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.8"
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection",
"doi-asserted-by": "crossref",
"first-page": "242",
"journal-title": "Clin Pharmacol Ther",
"key": "2025061108251181000_2025.06.09.25329141v1.9",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1877",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.10"
},
{
"DOI": "10.1128/AAC.00819-20",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.11",
"unstructured": "Jeon S , Ko M , Lee J , et al. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob Agents Chemother 2020; 64. Available at: /pmc/articles/PMC7318052/. Accessed 12 August 2024."
},
{
"DOI": "10.1038/s41467-020-18463-z",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.12",
"unstructured": "Shannon A , Selisko B , Le NTT , et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat Commun 2020; 11. Available at: /pmc/articles/PMC7499305/. Accessed 13 August 2024."
},
{
"DOI": "10.1016/j.antiviral.2020.104878",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.13",
"unstructured": "Pizzorno A , Padey B , Dubois J , et al. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antiviral Res 2020; 181. Available at: https://pubmed.ncbi.nlm.nih.gov/32679055/. Accessed 13 August 2024."
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.14",
"unstructured": "Choy KT , Wong AYL , Kaewpreedee P , et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 2020; 178. Available at: https://pubmed.ncbi.nlm.nih.gov/32251767/. Accessed 12 August 2024."
},
{
"DOI": "10.1128/mBio.01833-20",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.15",
"unstructured": "Hattori SI , Higshi-Kuwata N , Raghavaiah J , et al. GRL-0920 , an Indole Chloropyridinyl Ester, Completely Blocks SARS-CoV-2 Infection. mBio 2020; 11:1–16. Available at: https://pubmed.ncbi.nlm.nih.gov/32820005/. Accessed 12 August 2024."
},
{
"DOI": "10.1016/j.antiviral.2020.104955",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.16",
"unstructured": "Choi SW , Shin JS , Park SJ , et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res 2020; 184:104955. Available at: /pmc/articles/PMC7571425/. Accessed 16 August 2024."
},
{
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"first-page": "30",
"journal-title": "Cell Research",
"key": "2025061108251181000_2025.06.09.25329141v1.17",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2014441117",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.18"
},
{
"DOI": "10.1016/j.ebiom.2021.103595",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.19",
"unstructured": "Abdelnabi R , Foo CS , Kaptein SJF , et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine 2021; 72."
},
{
"DOI": "10.1038/s41467-021-21992-w",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.20",
"unstructured": "Driouich JS , Cochin M , Lingas G , et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat Commun 2021; 12."
},
{
"DOI": "10.1093/infdis/jiac135",
"article-title": "Favipiravir Treatment of Uncomplicated Influenza in Adults: Results of Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Trials",
"doi-asserted-by": "crossref",
"first-page": "1790",
"journal-title": "J Infect Dis",
"key": "2025061108251181000_2025.06.09.25329141v1.21",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.1111/cts.12827",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.22",
"unstructured": "Irie K , Nakagawa A , Fujita H , et al. Pharmacokinetics of Favipiravir in Critically Ill Patients With COVID-19. Clin Transl Sci 2020; 13:880. Available at: /pmc/articles/PMC7300626/. Accessed 12 August 2024."
},
{
"author": "Gülçebi İdriz Oğlu M",
"first-page": "3516–3522",
"journal-title": "Pharmacokinetic characterization of favipiravir in patients with COVID-19. Br J Clin Pharmacol",
"key": "2025061108251181000_2025.06.09.25329141v1.23",
"volume": "88",
"year": "2022"
},
{
"key": "2025061108251181000_2025.06.09.25329141v1.24",
"unstructured": "Arshad U , Pertinez H , Box H , et al. Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics. Clin Pharmacol Ther 2020; 108:775. Available at: /pmc/articles/PMC7280633/. Accessed 13 August 2024."
},
{
"DOI": "10.1093/jac/dkab135",
"article-title": "Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5′-triphosphate exposure to support posology for SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "2121",
"journal-title": "Journal of Antimicrobial Chemotherapy",
"key": "2025061108251181000_2025.06.09.25329141v1.25",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1128/AAC.01305-16",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.26",
"unstructured": "Madelain V , Guedj J , Mentre F , et al. Favipiravir Pharmacokinetics in Nonhuman Primates and Insights for Future Efficacy Studies of Hemorrhagic Fever Viruses. Antimicrob Agents Chemother 2017; 61. Available at: /pmc/articles/PMC5192134/. Accessed 13 August 2024."
},
{
"DOI": "10.1016/j.antiviral.2017.12.021",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.27"
},
{
"key": "2025061108251181000_2025.06.09.25329141v1.28",
"unstructured": "Sakurai T. A Phase I, Randomized, Double-blind, Placebo-controlled, Single Ascending-dose Study to Evaluate the Pharmacokinetics, Safety and Tolerability of Injectable Favipiravir in Healthy Subjects. Available at: https://jrct.niph.go.jp/en-latest-detail/jRCT2071210042. Accessed 13 January 2025."
},
{
"DOI": "10.1093/jac/dkab318",
"doi-asserted-by": "publisher",
"key": "2025061108251181000_2025.06.09.25329141v1.29"
},
{
"DOI": "10.1002/cpt.2463",
"article-title": "Phase 1 Trial of High-Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Clin Pharmacol Ther",
"key": "2025061108251181000_2025.06.09.25329141v1.30",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1080/10543406.2018.1535503",
"article-title": "Randomized dose-escalation designs for drug combination cancer trials with immunotherapy",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "J Biopharm Stat",
"key": "2025061108251181000_2025.06.09.25329141v1.31",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.1177/09622802241288348",
"article-title": "A seamless Phase I/II platform design with a time-to-event efficacy endpoint for potential COVID-19 therapies",
"doi-asserted-by": "crossref",
"first-page": "2115",
"journal-title": "Stat Methods Med Res",
"key": "2025061108251181000_2025.06.09.25329141v1.32",
"volume": "33",
"year": "2024"
},
{
"DOI": "10.1016/j.jpba.2023.115436",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.33",
"unstructured": "Challenger E , Penchala SD , Hale C , et al. Development and validation of an LC-MS/MS method for quantification of favipiravir in human plasma. J Pharm Biomed Anal 2023; 233:115436."
},
{
"DOI": "10.1002/psp4.12445",
"article-title": "Nonlinear Mixed-Effects Model Development and Simulation Using nlmixr and Related R Open-Source Packages",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "2025061108251181000_2025.06.09.25329141v1.34",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1002/psp4.12685",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"doi-asserted-by": "crossref",
"first-page": "1161",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "2025061108251181000_2025.06.09.25329141v1.35",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0005389",
"doi-asserted-by": "crossref",
"key": "2025061108251181000_2025.06.09.25329141v1.36",
"unstructured": "Nguyen THT , Guedj J , Anglaret X , et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis 2017; 11."
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2025.06.09.25329141"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Optimal dose and safety of intravenous favipiravir in hospitalised patients with SARS-CoV-2 infection: a Phase Ib, open-label, dose-escalating, randomised controlled study",
"type": "posted-content"
}