Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis
et al., Journal of Clinical Immunology, doi:10.1007/s10875-020-00911-6, Nov 2020
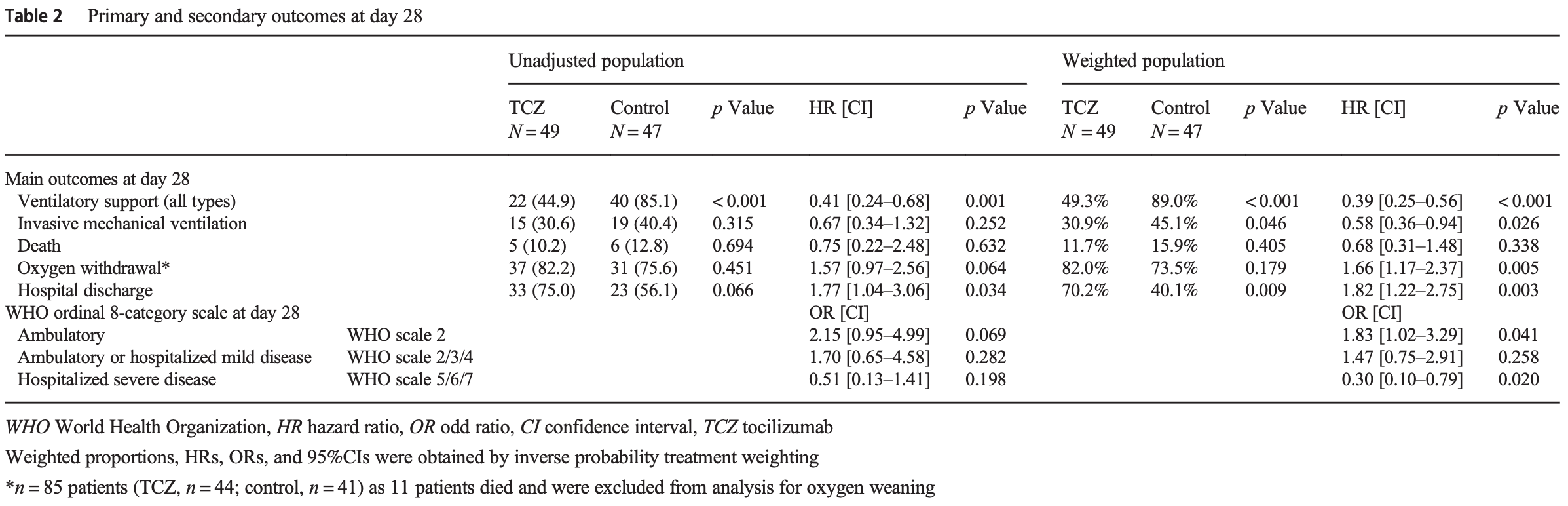
Prospective analysis of 96 hospitalized patients with severe worsening COVID-19 pneumonia showing that tocilizumab treatment was associated with reduced need for ventilatory support and shorter time to oxygen withdrawal, however there was no significant difference in mortality.
|
risk of death, 32.0% lower, HR 0.68, p = 0.34, treatment 49, control 47.
|
|
risk of mechanical ventilation, 42.0% lower, HR 0.58, p = 0.03, treatment 49, control 47.
|
|
risk of no hospital discharge, 45.1% lower, HR 0.55, p = 0.03, treatment 49, control 47, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Roumier et al., 14 Nov 2020, retrospective, France, peer-reviewed, mean age 60.0, 29 authors, study period 9 March, 2020 - 11 April, 2020.
Contact: f.ackermann@hopital-foch.com.
Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis
Journal of Clinical Immunology, doi:10.1007/s10875-020-00911-6
Background High levels of serum interleukin-6 (IL-6) correlate with disease severity in COVID-19. We hypothesized that tocilizumab (a recombinant humanized anti-IL-6 receptor) could improve outcomes in selected patients with severe worsening COVID-19 pneumonia and high inflammatory parameters. Methods The TOCICOVID study included a prospective cohort of patients aged 16-80 years with severe (requiring > 6 L/min of oxygen therapy to obtain Sp02 > 94%) rapidly deteriorating (increase by ≥ 3 L/min of oxygen flow within the previous 12 h) COVID-19 pneumonia with ≥ 5 days of symptoms and C-reactive protein levels > 40 mg/L. They entered a compassionate use program of treatment with intravenous tocilizumab (8 mg/kg with a maximum of 800 mg per infusion; and if needed a second infusion 24 to 72 h later). A control group was retrospectively selected with the same inclusion criteria. Outcomes were assessed at D28 using inverse probability of treatment weighted (IPTW) methodology. Results Among the 96 patients included (81% male, mean (SD) age: 60 (12.5) years), underlying conditions, baseline disease severity, and concomitant medications were broadly similar between the tocilizumab (n = 49) and the control (n = 47) groups. In the IPTW analysis, treatment with tocilizumab was associated with a reduced need for overall ventilatory support (49 vs. 89%, wHR: 0.39 [0.25-0.56]; p < 0.001). Albeit lacking statistical significance, there was a substantial trend towards a reduction of mechanical ventilation (31% vs. 45%;; p = 0.026). However, tocilizumab did not improve overall survival (wHR = 0.68 [0.31-1.748], p = 0.338). Among the 85 (89%) patients still alive at D28, patients treated with tocilizumab had a higher rate of oxygen withdrawal (82% vs. 73.5%,], p = 0.005), with a shorter delay before being
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10875-020-00911-6 . Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors' Contributions
References
Aouba, Baldolli, Geffray, Verdon, Bergot et al., Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series, Ann Rheum Dis
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Biran, Ip, Ahn, Go, Wang et al., Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study, Lancet Rheumatol
Campochiaro, Della-Torre, Cavalli, Luca, Ripa et al., Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study, Eur J Intern Med
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Cao, Wei, Zou, Jiang, Wang et al., Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial, J Allergy Clin Immunol
Capra, Rossi, Mattioli, Romanelli, Scarpazza et al., Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia, Eur J Intern Med
Cavalli, Luca, Campochiaro, Della-Torre, Ripa et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, Lancet Rheumatol
Cañete, Hernández, Sanmartí, Safety profile of biological therapies for treating rheumatoid arthritis, Expert Opin Biol Ther
Colaneri, Bogliolo, Valsecchi, Sacchi, Zuccaro et al., Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE), Microorganisms
Connors, Levy, COVID-19 and its implications for thrombosis and anticoagulation, Blood
De Luca, Cavalli, Campochiaro, Della-Torre, Angelillo et al., GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a singlecentre, prospective cohort study, Lancet Rheumatol
Dequin, Heming, Meziani, Plantefève, Voiriot et al., Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.16761
Figuero-Pérez, Olivares-Hernández, Escala-Cornejo, Terán-Brage, López-Gutiérrez et al., Anakinra as a potential alternative in the treatment of severe acute respiratory infection associated with SARS-CoV-2 refractory to tocilizumab, Reumatol Clin, doi:10.1016/j.reuma.2020.06.003
Galván-Román, Sc, Vallejo, Jiménez, Sánchez-Alonso et al., IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.09.018
Gao, Li, Han, Li, Wu et al., Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19, J Med Virol
Guaraldi, Meschiari, Cozzi-Lepri, Milic, Tonelli et al., Tocilizumab in patients with severe COVID-19: a retrospective cohort study, Lancet Rheumatol
Haukoos, Lewis, The propensity score, JAMA
Huet, Beaussier, Voisin, Jouveshomme, Dauriat et al., Anakinra for severe forms of COVID-19: a cohort study, Lancet Rheumatol
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Innes, Cook, Marks, Bataillard, Crossette-Thambiah et al., Ruxolitinib for tocilizumab-refractory severe COVID-19 infection, Br J Haematol, doi:10.1111/bjh.16979
Investigators, Angus, Derde, Al-Beidh, Annane et al., Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial, JAMA, doi:10.1001/jama.2020.17022
Mahévas, Tran, Roumier, Chabrol, Paule et al., Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, BMJ
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Netea, Rovina, Akinosoglou, Antoniadou, Antonakos, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host Microbe
Pan, Peto, Karim, Alejandria, Restrepo et al., Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results, medRxiv
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China, Clin Infect Dis
Radbel, Narayanan, Bhatt, Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report, Chest
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Somers, Eschenauer, Troost, Golob, Gandhi et al., Tocilizumab for treatment of mechanically ventilated patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciaa954
Sterne, Murthy, Diaz, Slutsky, Villar, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA, doi:10.1001/jama.2020.17023
Susen, Tacquard, Godon, Mansour, Nguyen et al., Traitement anticoagulant pour la prévention du risque thrombotique chez un patient hospitalisé avec COVID-19 et surveillance de l'hémostase propositions du GIHP et du GFHT
Tomazini, Maia, Cavalcanti, Berwanger, Rosa et al., Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial, JAMA, doi:10.1001/jama.2020.17021
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy, Autoimmun Rev
Wang, Zhang, Du, Du, Zhao, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Wu, Chen, Cai, Xia, Zhou et al., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med
Xu, Han, Li, Sun, Wang et al., Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Zhang, Li, Zhan, Wu, Yu et al., Analysis of serum cytokines in patients with severe acute respiratory syndrome, Infect Immun
Zhao, Zhang, Li, Ma, Gu et al., Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a s y s t e m a t i c r e v i e w a n d m e t a -a n a l y s i s, m e d R
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1007/s10875-020-00911-6",
"ISSN": [
"0271-9142",
"1573-2592"
],
"URL": "http://dx.doi.org/10.1007/s10875-020-00911-6",
"alternative-id": [
"911"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "15 July 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "3 November 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 November 2020"
},
{
"group": {
"label": "Compliance with Ethical Standards",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "MR: investigator of NCT04315298 trial which investigates the efficacy and safety of sarilumab (licensed by Sanofi) in hospitalized patients with COVID-19; non-financial support from Novartis Pharma SAS, Bristol Myers Squibb, and Swedish Orphan Biovitrum (outside the submitted work). HS: non-financial support from Oxyvie; grants for Foch Foundation, Fonds de dotation pour la recherche en santé respiratoire, and Philips Foundation (outside the submitted work). GG: non-financial support from Bard (outside the submitted work). YS: non-financial support from Astra Zeneca, Novartis Pharma, Bristol Myers Squibb, Sanofi Aventis France, Shire France, Chugai Pharma France, and Pfizer SAS (outside the submitted work). JLC: personal fees and non-financial support from Novartis, Boehringer Ingelheim, and Astra Zeneca; grants and other from LVL Air Liquide, outside the submitted work. JEK: none. MG: consulting fees from GlaxoSmithKline and Astra Zeneca (outside the submitted work). FA: investigator of NCT04315298 trial which investigates the efficacy and safety of sarilumab (licensed by Sanofi) in hospitalized patients with COVID-19. All other authors declare that they have no conflict of interest."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"affiliation": [],
"name": "on behalf of the Foch COVID-19 Study Group",
"sequence": "first"
},
{
"affiliation": [],
"family": "Roumier",
"given": "Mathilde",
"sequence": "first"
},
{
"affiliation": [],
"family": "Paule",
"given": "Romain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vallée",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rohmer",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ballester",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brun",
"given": "Anne-Laure",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cerf",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chabi",
"given": "Marie-Laure",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chinet",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colombier",
"given": "Marie-Alice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farfour",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fourn",
"given": "Erwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Géri",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khau",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marroun",
"given": "Ibrahim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponsoye",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roux",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvator",
"given": "Hélène",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schoindre",
"given": "Yoland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Si Larbi",
"given": "Anne-Gaëlle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tchérakian",
"given": "Colas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasse",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verrat",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zuber",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Couderc",
"given": "Louis-Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kahn",
"given": "Jean-Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Groh",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ackermann",
"given": "Félix",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Immunology",
"container-title-short": "J Clin Immunol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
11,
14
]
],
"date-time": "2020-11-14T01:02:28Z",
"timestamp": 1605315748000
},
"deposited": {
"date-parts": [
[
2021,
2,
3
]
],
"date-time": "2021-02-03T16:11:19Z",
"timestamp": 1612368679000
},
"indexed": {
"date-parts": [
[
2025,
5,
27
]
],
"date-time": "2025-05-27T18:26:17Z",
"timestamp": 1748370377793
},
"is-referenced-by-count": 36,
"issue": "2",
"issued": {
"date-parts": [
[
2020,
11,
14
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
14
]
],
"date-time": "2020-11-14T00:00:00Z",
"timestamp": 1605312000000
}
},
{
"URL": "http://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
14
]
],
"date-time": "2020-11-14T00:00:00Z",
"timestamp": 1605312000000
}
}
],
"link": [
{
"URL": "http://link.springer.com/content/pdf/10.1007/s10875-020-00911-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/article/10.1007/s10875-020-00911-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/content/pdf/10.1007/s10875-020-00911-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "303-314",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2020,
11,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
11,
14
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1093/cid/ciaa248",
"author": "C Qin",
"doi-asserted-by": "publisher",
"first-page": "762",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "911_CR1",
"unstructured": "Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020006000",
"author": "JM Connors",
"doi-asserted-by": "publisher",
"first-page": "2033",
"issue": "23",
"journal-title": "Blood",
"key": "911_CR2",
"unstructured": "Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40.",
"volume": "135",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "911_CR3",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "911_CR4",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1128/IAI.72.8.4410-4415.2004",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "4410",
"issue": "8",
"journal-title": "Infect Immun",
"key": "911_CR5",
"unstructured": "Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72(8):4410–5.",
"volume": "72",
"year": "2004"
},
{
"DOI": "10.1002/jmv.25770",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"first-page": "791",
"issue": "7",
"journal-title": "J Med Virol",
"key": "911_CR6",
"unstructured": "Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–6.",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"author": "X Xu",
"doi-asserted-by": "publisher",
"first-page": "10970",
"issue": "20",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "911_CR7",
"unstructured": "Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–5.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"author": "EJ Giamarellos-Bourboulis",
"doi-asserted-by": "publisher",
"first-page": "992",
"issue": "6",
"journal-title": "Cell Host Microbe",
"key": "911_CR8",
"unstructured": "Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3.",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1080/14712598.2017.1346078",
"author": "JD Cañete",
"doi-asserted-by": "publisher",
"first-page": "1089",
"issue": "9",
"journal-title": "Expert Opin Biol Ther",
"key": "911_CR9",
"unstructured": "Cañete JD, Hernández MV, Sanmartí R. Safety profile of biological therapies for treating rheumatoid arthritis. Expert Opin Biol Ther. 2017;17(9):1089–103.",
"volume": "17",
"year": "2017"
},
{
"key": "911_CR10",
"unstructured": "Susen S, Tacquard CA, Godon A, Mansour A, Nguyen P, Godier A, et al. Traitement anticoagulant pour la prévention du risque thrombotique chez un patient hospitalisé avec COVID-19 et surveillance de l’hémostase propositions du GIHP et du GFHT. https://www.portailvasculaire.fr/sites/default/files/docs/covid-19_gihp-gfht-3_avril_final.pdf."
},
{
"key": "911_CR11",
"unstructured": "SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. Recommandations d’experts portant sur la prise en charge en réanimation des patients en période d’épidémie à SARS-CoV2. Avr. 2020. https://www.srlf.org/wp-content/uploads/2020/04/RFE-COVID_V4.pdf."
},
{
"DOI": "10.1001/jama.2015.13480",
"author": "JS Haukoos",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "15",
"journal-title": "JAMA.",
"key": "911_CR12",
"unstructured": "Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–8.",
"volume": "314",
"year": "2015"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"author": "C Wu",
"doi-asserted-by": "publisher",
"first-page": "934",
"issue": "7",
"journal-title": "JAMA Intern Med",
"key": "911_CR13",
"unstructured": "Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.",
"volume": "180",
"year": "2020"
},
{
"key": "911_CR14",
"unstructured": "Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020;2020.03.17.20037572."
},
{
"DOI": "10.1056/NEJMoa2001282",
"author": "B Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "911_CR15",
"unstructured": "Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1844",
"author": "M Mahévas",
"doi-asserted-by": "publisher",
"first-page": "m1844",
"journal-title": "BMJ.",
"key": "911_CR16",
"unstructured": "Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"author": "IF-N Hung",
"doi-asserted-by": "publisher",
"first-page": "1695",
"issue": "10238",
"journal-title": "Lancet.",
"key": "911_CR17",
"unstructured": "Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704.",
"volume": "395",
"year": "2020"
},
{
"key": "911_CR18",
"unstructured": "Pan H, Peto R, Abdool Karim Q, Alejandria M, Henao Restrepo AM, Hernandez Garcia C, et al. Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results. medRxiv. 2020;2020.10.15.20209817."
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"issue": "10236",
"journal-title": "Lancet.",
"key": "911_CR19",
"unstructured": "Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "911_CR20",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;NEJMoa2007764:1813–26. https://doi.org/10.1056/NEJMoa2007764.",
"volume": "NEJMoa2007764",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"author": "X Yang",
"doi-asserted-by": "publisher",
"first-page": "475",
"issue": "5",
"journal-title": "Lancet Respir Med",
"key": "911_CR21",
"unstructured": "Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"author": "P Toniati",
"doi-asserted-by": "publisher",
"first-page": "102568",
"issue": "7",
"journal-title": "Autoimmun Rev",
"key": "911_CR22",
"unstructured": "Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.009",
"author": "R Capra",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Eur J Intern Med",
"key": "911_CR23",
"unstructured": "Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–5.",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.021",
"author": "C Campochiaro",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Eur J Intern Med",
"key": "911_CR24",
"unstructured": "Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9.",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30173-9",
"author": "G Guaraldi",
"doi-asserted-by": "publisher",
"first-page": "e474",
"issue": "8",
"journal-title": "Lancet Rheumatol",
"key": "911_CR25",
"unstructured": "Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–84.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa954",
"doi-asserted-by": "publisher",
"key": "911_CR26",
"unstructured": "Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020;ciaa954. https://doi.org/10.1093/cid/ciaa954."
},
{
"DOI": "10.1016/S2665-9913(20)30277-0",
"author": "N Biran",
"doi-asserted-by": "publisher",
"first-page": "e603",
"issue": "10",
"journal-title": "Lancet Rheumatol",
"key": "911_CR27",
"unstructured": "Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–12.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.reuma.2020.06.003",
"author": "L Figuero-Pérez",
"doi-asserted-by": "publisher",
"first-page": "30142",
"issue": "20",
"journal-title": "Reumatol Clin",
"key": "911_CR28",
"unstructured": "Figuero-Pérez L, Olivares-Hernández A, Escala-Cornejo RA, Terán-Brage E, López-Gutiérrez Á, Cruz-Hernández JJ. Anakinra as a potential alternative in the treatment of severe acute respiratory infection associated with SARS-CoV-2 refractory to tocilizumab. Reumatol Clin. 2020;S1699-258X(20):30142–X. https://doi.org/10.1016/j.reuma.2020.06.003.",
"volume": "S1699-258X",
"year": "2020"
},
{
"DOI": "10.1111/bjh.16979",
"doi-asserted-by": "publisher",
"key": "911_CR29",
"unstructured": "Innes AJ, Cook LB, Marks S, Bataillard E, Crossette-Thambiah C, Sivasubramaniam G, et al. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br J Haematol. 2020;190. https://doi.org/10.1111/bjh.16979."
},
{
"DOI": "10.1016/j.chest.2020.04.024",
"author": "J Radbel",
"doi-asserted-by": "publisher",
"first-page": "e15",
"issue": "1",
"journal-title": "Chest.",
"key": "911_CR30",
"unstructured": "Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158(1):e15–9.",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.3390/microorganisms8050695",
"doi-asserted-by": "crossref",
"key": "911_CR31",
"unstructured": "Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):695."
},
{
"key": "911_CR32",
"unstructured": "Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra® in hospitalised patients with severe COVID-19 associated pneumonia. 2020. Available at: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. Accessed October 10th 2020."
},
{
"DOI": "10.5005/jsd-10-1-iv",
"doi-asserted-by": "crossref",
"key": "911_CR33",
"unstructured": "Sanofi provides update on Kevzara® (sarilumab) phase 3 trial in severe and critically ill COVID-19 patients outside the U.S. 2020. Available at: https://www.sanofigenzyme.com/about-us/newsroom/2020/2020-09-01-00-00-00. Accessed October 10th 2020."
},
{
"DOI": "10.1016/j.jaci.2020.09.018",
"author": "JM Galván-Román",
"doi-asserted-by": "publisher",
"first-page": "31329",
"issue": "20",
"journal-title": "J Allergy Clin Immunol",
"key": "911_CR34",
"unstructured": "Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol. 2020;S0091–6749(20):31329–4. https://doi.org/10.1016/j.jaci.2020.09.018.",
"volume": "S0091–6749",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217706",
"author": "A Aouba",
"doi-asserted-by": "publisher",
"first-page": "1381",
"issue": "10",
"journal-title": "Ann Rheum Dis",
"key": "911_CR35",
"unstructured": "Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–2.",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30164-8",
"author": "T Huet",
"doi-asserted-by": "publisher",
"first-page": "e393",
"issue": "7",
"journal-title": "Lancet Rheumatol",
"key": "911_CR36",
"unstructured": "Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–400.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"author": "G Cavalli",
"doi-asserted-by": "publisher",
"first-page": "e325",
"issue": "6",
"journal-title": "Lancet Rheumatol",
"key": "911_CR37",
"unstructured": "Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–31.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.05.019",
"author": "Y Cao",
"doi-asserted-by": "publisher",
"first-page": "137",
"issue": "1",
"journal-title": "J Allergy Clin Immunol",
"key": "911_CR38",
"unstructured": "Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146.e3.",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30170-3",
"author": "G De Luca",
"doi-asserted-by": "publisher",
"first-page": "e465",
"issue": "8",
"journal-title": "Lancet Rheumatol",
"key": "911_CR39",
"unstructured": "De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2(8):e465–73.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17022",
"author": "DC Angus",
"doi-asserted-by": "publisher",
"first-page": "1317",
"issue": "13",
"journal-title": "JAMA.",
"key": "911_CR40",
"unstructured": "Writing Committee for the REMAP-CAP Investigators, Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29. https://doi.org/10.1001/jama.2020.17022.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16761",
"author": "P-F Dequin",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "13",
"journal-title": "JAMA.",
"key": "911_CR41",
"unstructured": "Dequin P-F, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1–9. https://doi.org/10.1001/jama.2020.16761.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17021",
"author": "BM Tomazini",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "13",
"journal-title": "JAMA.",
"key": "911_CR42",
"unstructured": "Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1–11. https://doi.org/10.1001/jama.2020.17021.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "911_CR43",
"unstructured": "RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436."
},
{
"DOI": "10.1001/jama.2020.17023",
"author": "JAC Sterne",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "13",
"journal-title": "JAMA",
"key": "911_CR44",
"unstructured": "WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1–13. https://doi.org/10.1001/jama.2020.17023.",
"volume": "324",
"year": "2020"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "http://link.springer.com/10.1007/s10875-020-00911-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "41"
}