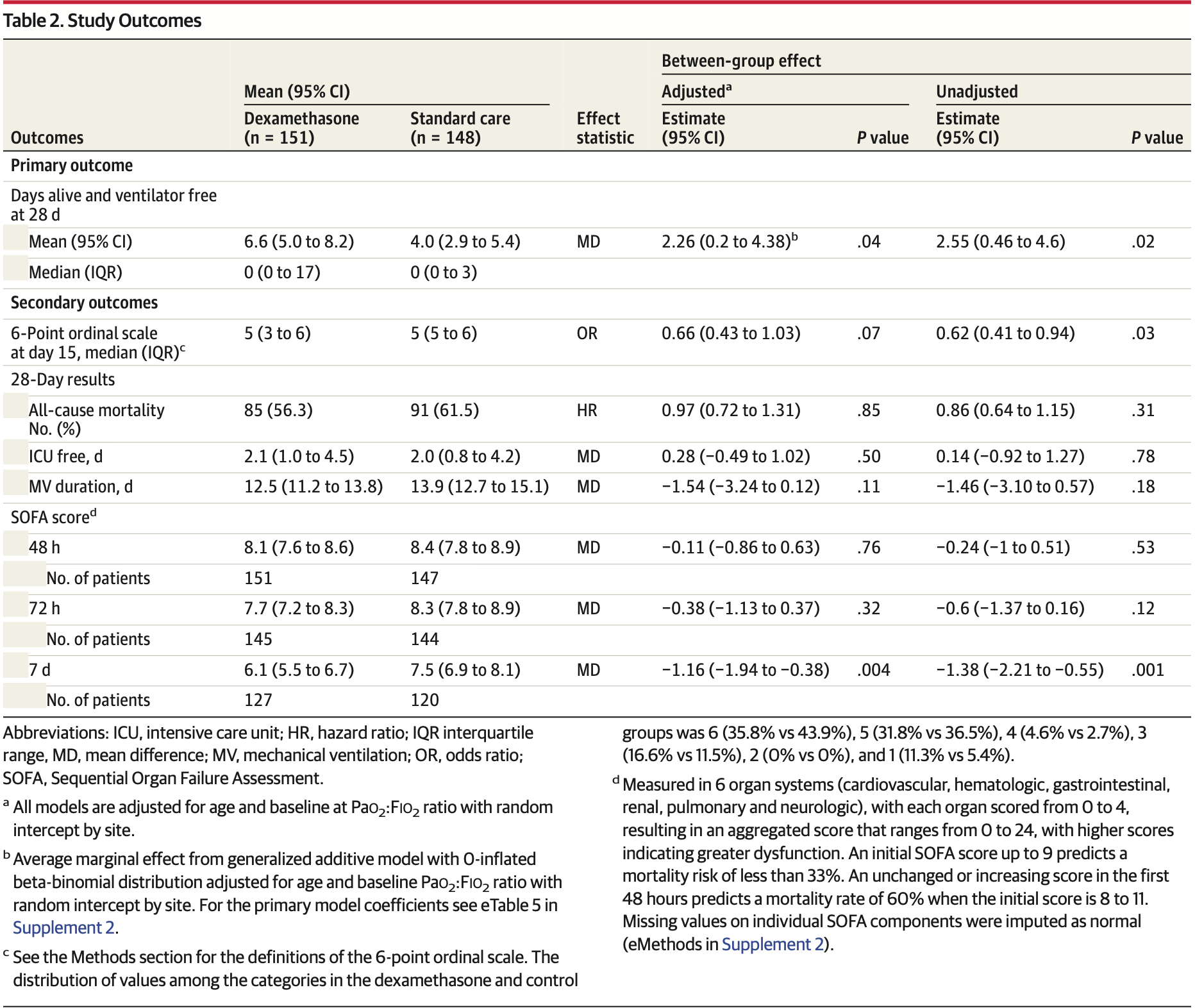
Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19
et al., JAMA, doi:10.1001/jama.2020.17021, CoDEX, NCT04327401, Oct 2020
RCT 299 patients with moderate or severe COVID-19-related ARDS showing increased ventilator-free days with dexamethasone treatment. There was no significant difference in 28-day mortality (56.3% vs 61.5%), ICU-free days, or mechanical ventilation duration.
|
risk of death, 3.0% lower, HR 0.97, p = 0.85, treatment 85 of 151 (56.3%), control 91 of 148 (61.5%), NNT 19, adjusted per study, day 28.
|
|
6-point scale, 34.0% lower, OR 0.66, p = 0.07, treatment 151, control 148, day 15, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tomazini et al., 6 Oct 2020, Randomized Controlled Trial, Brazil, peer-reviewed, 34 authors, study period 17 April, 2020 - 23 June, 2020, trial NCT04327401 (history) (CoDEX).
Contact: luciano.azevedo@hsl.org.br, angusdc@upmc.edu.
Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19
JAMA, doi:10.1001/jama.2020.17021
Brazil III Investigators IMPORTANCE Acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19) is associated with substantial mortality and use of health care resources. Dexamethasone use might attenuate lung injury in these patients. OBJECTIVE To determine whether intravenous dexamethasone increases the number of ventilator-free days among patients with COVID-19-associated ARDS. DESIGN, SETTING, AND PARTICIPANTS Multicenter, randomized, open-label, clinical trial conducted in 41 intensive care units (ICUs) in Brazil. Patients with COVID-19 and moderate to severe ARDS, according to the Berlin definition, were enrolled from April 17 to June 23, 2020. Final follow-up was completed on July 21, 2020. The trial was stopped early following publication of a related study before reaching the planned sample size of 350 patients. INTERVENTIONS Twenty mg of dexamethasone intravenously daily for 5 days, 10 mg of dexamethasone daily for 5 days or until ICU discharge, plus standard care (n =151) or standard care alone (n = 148).
MAIN OUTCOMES AND MEASURES The primary outcome was ventilator-free days during the first 28 days, defined as being alive and free from mechanical ventilation. Secondary outcomes were all-cause mortality at 28 days, clinical status of patients at day 15 using a 6-point ordinal scale (ranging from 1, not hospitalized to 6, death), ICU-free days during the first 28 days, mechanical ventilation duration at 28 days, and Sequential Organ Failure Assessment (SOFA) scores (range, 0-24, with higher scores indicating greater organ dysfunction) at 48 hours, 72 hours, and 7 days. RESULTS A total of 299 patients (mean [SD] age, 61 [14] years; 37% women) were enrolled and all completed follow-up. Patients randomized to the dexamethasone group had a mean 6.6 ventilator-free days (95% CI, 5.0-8.2) during the first 28 days vs 4.0 ventilator-free days (95% CI, 2.9-5.4) in the standard care group (difference, 2.26; 95% CI, 0.2-4.38; P = .04). At 7 days, patients in the dexamethasone group had a mean SOFA score of 6.1 (95% CI, 5.5-6.7) vs 7.5 (95% CI, 6.9-8.1) in the standard care group (difference, -1.16; 95% CI, -1.94 to -0.38; P = .004). There was no significant difference in the prespecified secondary outcomes of all-cause mortality at 28 days, ICU-free days during the first 28 days, mechanical ventilation duration at 28 days, or the 6-point ordinal scale at 15 days. Thirty-three patients (21.9%) in the dexamethasone group vs 43 (29.1%) in the standard care group experienced secondary infections, 47 (31.1%) vs 42 (28.3%) needed insulin for glucose control, and 5 (3.3%) vs 9 (6.1%) experienced other serious adverse events. CONCLUSIONS AND RELEVANCE Among patients with COVID-19 and moderate or severe ARDS, use of intravenous dexamethasone plus standard care compared with standard care alone resulted in a statistically significant increase in the number of ventilator-free days (days alive and free of mechanical ventilation)..
Author Contributions: Drs Tomazini and Azevedo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Mr Damiani conducted and is responsible for the data analysis.
Concept and design:
References
Ackermann, Verleden, Kuehnel, Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19, N Engl J Med, doi:10.1056/NEJMoa2015432
Annane, Pastores, Rochwerg, Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients, I: Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017, Crit Care Med, doi:10.1097/CCM.0000000000002737
Arabi, Mandourah, Hameed, Corticosteroid Therapy for critically ill patients with Middle East respiratory syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.201706-1172OC
Blenkinsop, Parmar, Choodari-Oskooei, Assessing the impact of efficacy stopping rules on the error rates under the multi-arm multi-stage framework, Clin Trials, doi:10.1177/1740774518823551
Béduneau, Pham, Schortgen, Weaning according to a New Definition) Study Group and the REVA (Réseau Européen de Recherche en Ventilation Artificielle) Network ‡. Epidemiology of weaning outcome according to a new definition: the WIND Study, Am J Respir Crit Care Med, doi:10.1164/rccm.201602-0320OC
Cao, Gao, Zhou, Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia, Crit Care Med, doi:10.1097/CCM.0000000000001616
Cavalcanti, Suzumura, Laranjeira, Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial, JAMA, doi:10.1001/jama.2017.14171?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2020.17021
Docherty, Harrison, Green, ISARIC4C investigators. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Fernandes, Morais, Zung, Machado, Azevedo et al., Statistical analysis: Tomazini
Ferrando, Suarez-Sipmann, Mellado-Artigas, COVID-19 Spanish ICU Network. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS, Intensive Care Med, doi:10.1007/s00134-020-06192-2
Grasselli, Greco, Zanella, Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy, JAMA Intern Med, doi:10.1001/jamainternmed.2020.3539?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2020.17021
Grasselli, Zangrillo, Zanella, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy, JAMA, doi:10.1001/jama.2020.5394?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2020.17021
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Lee, Chan, Hui, Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients, J Clin Virol, doi:10.1016/j.jcv.2004.07.006
Lehmann, Abrera, Nonparametrics: Statistical Methods Based on Ranks
Metnitz, Moreno, Almeida, SAPS 3 Investigators. SAPS 3-from evaluation of the patient to evaluation of the intensive care unit, I: objectives, methods and cohort description, Intensive Care Med, doi:10.1007/s00134-005-2762-6
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Moreno, Metnitz, Almeida, SAPS 3 Investigators. SAPS 3-from evaluation of the patient to evaluation of the intensive care unit, II: development of a prognostic model for hospital mortality at ICU admission, Intensive Care Med, doi:10.1007/s00134-005-2763-5
Neto, Barbas, Simonis, Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study, Lancet Respir Med, doi:10.1016/S2213-2600(16)30305-8
Ni, Chen, Sun, Liang, Liang, The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis, Crit Care, doi:10.1186/s13054-019-2395-8
Qin, Zhou, Hu, Dysregulation of Immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China, Clin Infect Dis, doi:10.1093/cid/ciaa248
Ranieri, Rubenfeld, Thompson, ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition, JAMA, doi:10.1001/jama.2012.5669?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2020.17021
Rhen, Cidlowski, Antiinflammatory action of glucocorticoids-new mechanisms for old drugs, N Engl J Med, doi:10.1056/NEJMra050541
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA, doi:10.1001/jama.2020.6775?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2020.17021
Steinberg, Hudson, Goodman, ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome, N Engl J Med, doi:10.1056/NEJMoa051693
Tomazini, Bueno, Silva, Baldassare, Moura et al., Santa Casa de Misericórdia de Passos
Tomazini, Maia, Bueno, COVID-19-associated ARDS treated with DEXamethasone (CoDEX): study design and rationale for a randomized trial, Rev Bras Ter Intensiva
Villar, Ferrando, Martínez, Dexamethasone in ARDS Network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(19)30417-5
Wang, Lu, Li, Clinical course and outcomes of 344 intensive care patients with COVID-19, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0736LE
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhu, Zhang, Wang, China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1001/jama.2020.17021",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2020.17021",
"author": [
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
},
{
"name": "Departamento de Cirurgia, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Tomazini",
"given": "Bruno M.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
},
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
}
],
"family": "Maia",
"given": "Israel S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
},
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
}
],
"family": "Cavalcanti",
"given": "Alexandre B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil"
}
],
"family": "Berwanger",
"given": "Otavio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "Hospital Moinhos de Vento, Porto Alegre, Brazil"
}
],
"family": "Rosa",
"given": "Regis G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "BP–A Beneficência Portuguesa de São Paulo, São Paulo, Brazil"
}
],
"family": "Veiga",
"given": "Viviane C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "International Research Center, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil"
}
],
"family": "Avezum",
"given": "Alvaro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brazilian Clinical Research Institute, São Paulo, Brazil"
},
{
"name": "Duke University Medical Center, Duke Clinical Research Institute, Durham, North Carolina"
}
],
"family": "Lopes",
"given": "Renato D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
}
],
"family": "Bueno",
"given": "Flavia R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
}
],
"family": "Silva",
"given": "Maria Vitoria A. O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
}
],
"family": "Baldassare",
"given": "Franca P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
},
{
"name": "UTI Respiratória, Instituto do Coração (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Costa",
"given": "Eduardo L. V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
}
],
"family": "Moura",
"given": "Ricardo A. B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
}
],
"family": "Honorato",
"given": "Michele O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
},
{
"name": "Departamento de Cardiopneumologia, Instituto do Coração (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Costa",
"given": "Andre N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
}
],
"family": "Damiani",
"given": "Lucas P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
},
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "Hospital de Clinicas de Porto Alegre, Rio Grande do Sul, Brazil"
}
],
"family": "Lisboa",
"given": "Thiago",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
}
],
"family": "Kawano-Dourado",
"given": "Letícia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCor Research Institute, São Paulo, Brazil"
},
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
}
],
"family": "Zampieri",
"given": "Fernando G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil"
},
{
"name": "Hospital Vila Santa Catarina, São Paulo, Brazil"
}
],
"family": "Olivato",
"given": "Guilherme B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Estadual do Cérebro Paulo Niemeyer, Rio de Janeiro, Brazil"
},
{
"name": "Laboratorio de Medicina Intensiva, Instituto Nacional de Infectologia, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil"
}
],
"family": "Righy",
"given": "Cassia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Barretos Cancer Hospital, Barretos, Brazil"
}
],
"family": "Amendola",
"given": "Cristina P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departamento de Cirurgia, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
},
{
"name": "Intensive Care Unit, AC Camargo Cancer Center, São Paulo, Brazil"
}
],
"family": "Roepke",
"given": "Roberta M. L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UTI Respiratória, Instituto do Coração (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Freitas",
"given": "Daniela H. M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
},
{
"name": "UTI 09DN, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Forte",
"given": "Daniel N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "Anesthesiology, Pain, and Intensive Care Department, Federal University of São Paulo, São Paulo, Brazil"
}
],
"family": "Freitas",
"given": "Flávio G. R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Mario Covas, FMABC, Santo Andre, Brazil"
}
],
"family": "Fernandes",
"given": "Caio C. F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Samaritano Paulista, São Paulo, Brazil"
}
],
"family": "Melro",
"given": "Livia M. G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Evangélico de Vila Velha, Vila Velha, Brazil"
}
],
"family": "Junior",
"given": "Gedealvares F. S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aché Laboratórios Farmacêuticos, São Paulo, Brazil"
}
],
"family": "Morais",
"given": "Douglas Costa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aché Laboratórios Farmacêuticos, São Paulo, Brazil"
}
],
"family": "Zung",
"given": "Stevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "Anesthesiology, Pain, and Intensive Care Department, Federal University of São Paulo, São Paulo, Brazil"
}
],
"family": "Machado",
"given": "Flávia R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Sírio-Libanês, São Paulo, Brazil"
},
{
"name": "Brazilian Research in Intensive Care Network (BRICNet), São Paulo, Brazil"
},
{
"name": "Disciplina de Emergências Clínicas, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil"
}
],
"family": "Azevedo",
"given": "Luciano C. P.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "COALITION COVID-19 Brazil III Investigators",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
2
]
],
"date-time": "2020-09-02T17:15:22Z",
"timestamp": 1599066922000
},
"deposited": {
"date-parts": [
[
2020,
10,
6
]
],
"date-time": "2020-10-06T15:01:48Z",
"timestamp": 1601996508000
},
"indexed": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T16:35:08Z",
"timestamp": 1749227708021
},
"is-referenced-by-count": 1034,
"issue": "13",
"issued": {
"date-parts": [
[
2020,
10,
6
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2020,
10,
6
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2770277/jama_tomazini_2020_oi_200102_1601660270.28279.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1307",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2020,
10,
6
]
]
},
"published-print": {
"date-parts": [
[
2020,
10,
6
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019.",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "joi200102r1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area.",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "joi200102r3",
"volume": "323",
"year": "2020"
},
{
"article-title": "Features of 20?133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study.",
"author": "Docherty",
"journal-title": "BMJ",
"key": "joi200102r4"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy.",
"author": "Grasselli",
"doi-asserted-by": "publisher",
"first-page": "1574",
"issue": "16",
"journal-title": "JAMA",
"key": "joi200102r5",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19.",
"author": "Ackermann",
"doi-asserted-by": "publisher",
"first-page": "120",
"issue": "2",
"journal-title": "N Engl J Med",
"key": "joi200102r6",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19.",
"author": "Moore",
"doi-asserted-by": "publisher",
"first-page": "473",
"issue": "6490",
"journal-title": "Science",
"key": "joi200102r7",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa248",
"article-title": "Dysregulation of Immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China.",
"author": "Qin",
"doi-asserted-by": "publisher",
"first-page": "762",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "joi200102r8",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1056/NEJMra050541",
"article-title": "Antiinflammatory action of glucocorticoids—new mechanisms for old drugs.",
"author": "Rhen",
"doi-asserted-by": "publisher",
"first-page": "1711",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "joi200102r9",
"volume": "353",
"year": "2005"
},
{
"DOI": "10.1056/NEJMoa051693",
"article-title": "Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome.",
"author": "Steinberg",
"doi-asserted-by": "publisher",
"first-page": "1671",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "joi200102r10",
"volume": "354",
"year": "2006"
},
{
"DOI": "10.1016/S2213-2600(19)30417-5",
"article-title": "Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial.",
"author": "Villar",
"doi-asserted-by": "publisher",
"first-page": "267",
"issue": "3",
"journal-title": "Lancet Respir Med",
"key": "joi200102r11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2004.07.006",
"article-title": "Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients.",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "304",
"issue": "4",
"journal-title": "J Clin Virol",
"key": "joi200102r12",
"volume": "31",
"year": "2004"
},
{
"DOI": "10.1164/rccm.201706-1172OC",
"article-title": "Corticosteroid Therapy for critically ill patients with Middle East respiratory syndrome.",
"author": "Arabi",
"doi-asserted-by": "publisher",
"first-page": "757",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi200102r13",
"volume": "197",
"year": "2018"
},
{
"DOI": "10.1186/s13054-019-2395-8",
"article-title": "The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis.",
"author": "Ni",
"doi-asserted-by": "publisher",
"first-page": "99",
"issue": "1",
"journal-title": "Crit Care",
"key": "joi200102r14",
"volume": "23",
"year": "2019"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary report.",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "joi200102r15"
},
{
"article-title": "COVID-19–associated ARDS treated with DEXamethasone (CoDEX): study design and rationale for a randomized trial.",
"author": "Tomazini",
"journal-title": "Rev Bras Ter Intensiva",
"key": "joi200102r16"
},
{
"article-title": "Acute respiratory distress syndrome: the Berlin definition.",
"author": "Ranieri",
"first-page": "2526",
"issue": "23",
"journal-title": "JAMA",
"key": "joi200102r17",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners.",
"author": "Harris",
"doi-asserted-by": "crossref",
"journal-title": "J Biomed Inform",
"key": "joi200102r18",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1097/CCM.0000000000002737",
"article-title": "Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients, I: Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017.",
"author": "Annane",
"doi-asserted-by": "publisher",
"first-page": "2078",
"issue": "12",
"journal-title": "Crit Care Med",
"key": "joi200102r19",
"volume": "45",
"year": "2017"
},
{
"DOI": "10.1164/rccm.201602-0320OC",
"article-title": "Epidemiology of weaning outcome according to a new definition: the WIND Study.",
"author": "Béduneau",
"doi-asserted-by": "publisher",
"first-page": "772",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi200102r20",
"volume": "195",
"year": "2017"
},
{
"DOI": "10.1001/jama.2017.14171",
"article-title": "Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial.",
"author": "Cavalcanti",
"doi-asserted-by": "publisher",
"first-page": "1335",
"issue": "14",
"journal-title": "JAMA",
"key": "joi200102r22",
"volume": "318",
"year": "2017"
},
{
"DOI": "10.1177/1740774518823551",
"article-title": "Assessing the impact of efficacy stopping rules on the error rates under the multi-arm multi-stage framework.",
"author": "Blenkinsop",
"doi-asserted-by": "publisher",
"first-page": "132",
"issue": "2",
"journal-title": "Clin Trials",
"key": "joi200102r24",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1016/S2213-2600(16)30305-8",
"article-title": "Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study.",
"author": "Neto",
"doi-asserted-by": "publisher",
"first-page": "882",
"issue": "11",
"journal-title": "Lancet Respir Med",
"key": "joi200102r25",
"volume": "4",
"year": "2016"
},
{
"article-title": "Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS.",
"author": "Ferrando",
"journal-title": "Intensive Care Med",
"key": "joi200102r26"
},
{
"DOI": "10.1007/s00134-005-2763-5",
"article-title": "SAPS 3—from evaluation of the patient to evaluation of the intensive care unit, II: development of a prognostic model for hospital mortality at ICU admission.",
"author": "Moreno",
"doi-asserted-by": "publisher",
"first-page": "1345",
"issue": "10",
"journal-title": "Intensive Care Med",
"key": "joi200102r27",
"volume": "31",
"year": "2005"
},
{
"DOI": "10.1007/s00134-005-2762-6",
"article-title": "SAPS 3—from evaluation of the patient to evaluation of the intensive care unit, I: objectives, methods and cohort description.",
"author": "Metnitz",
"doi-asserted-by": "publisher",
"first-page": "1336",
"issue": "10",
"journal-title": "Intensive Care Med",
"key": "joi200102r28",
"volume": "31",
"year": "2005"
},
{
"article-title": "Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy.",
"author": "Grasselli",
"journal-title": "JAMA Intern Med",
"key": "joi200102r30"
},
{
"DOI": "10.1164/rccm.202003-0736LE",
"article-title": "Clinical course and outcomes of 344 intensive care patients with COVID-19.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1430",
"issue": "11",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi200102r31",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "joi200102r32",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000001616",
"article-title": "Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "e318",
"issue": "6",
"journal-title": "Crit Care Med",
"key": "joi200102r33",
"volume": "44",
"year": "2016"
},
{
"author": "Lehmann",
"key": "joi200102r23",
"volume-title": "Nonparametrics: Statistical Methods Based on Ranks",
"year": "1975"
},
{
"key": "joi200102r2",
"unstructured": "World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19. Posted March 11, 2020. Accessed March 25, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020"
},
{
"key": "joi200102r21",
"unstructured": "World Health Organization. COVID-19 therapeutic trial synopsis. Draft February 18, 2020. Accessed July 28, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf"
},
{
"key": "joi200102r29",
"unstructured": "Uso de Supporte na Unidade de Principais Desfechos—Internaçõis em UTI Adulto com Desfecho Hospitalar Atribuísdo. UTIs Brasileiras. Updated August 19, 2020. Accessed July 31, 2020. http://www.utisbrasileiras.com.br/sari-covid-19/benchmarking-covid-19/?"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2770277"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"The CoDEX Randomized Clinical Trial"
],
"title": "Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19",
"type": "journal-article",
"volume": "324"
}