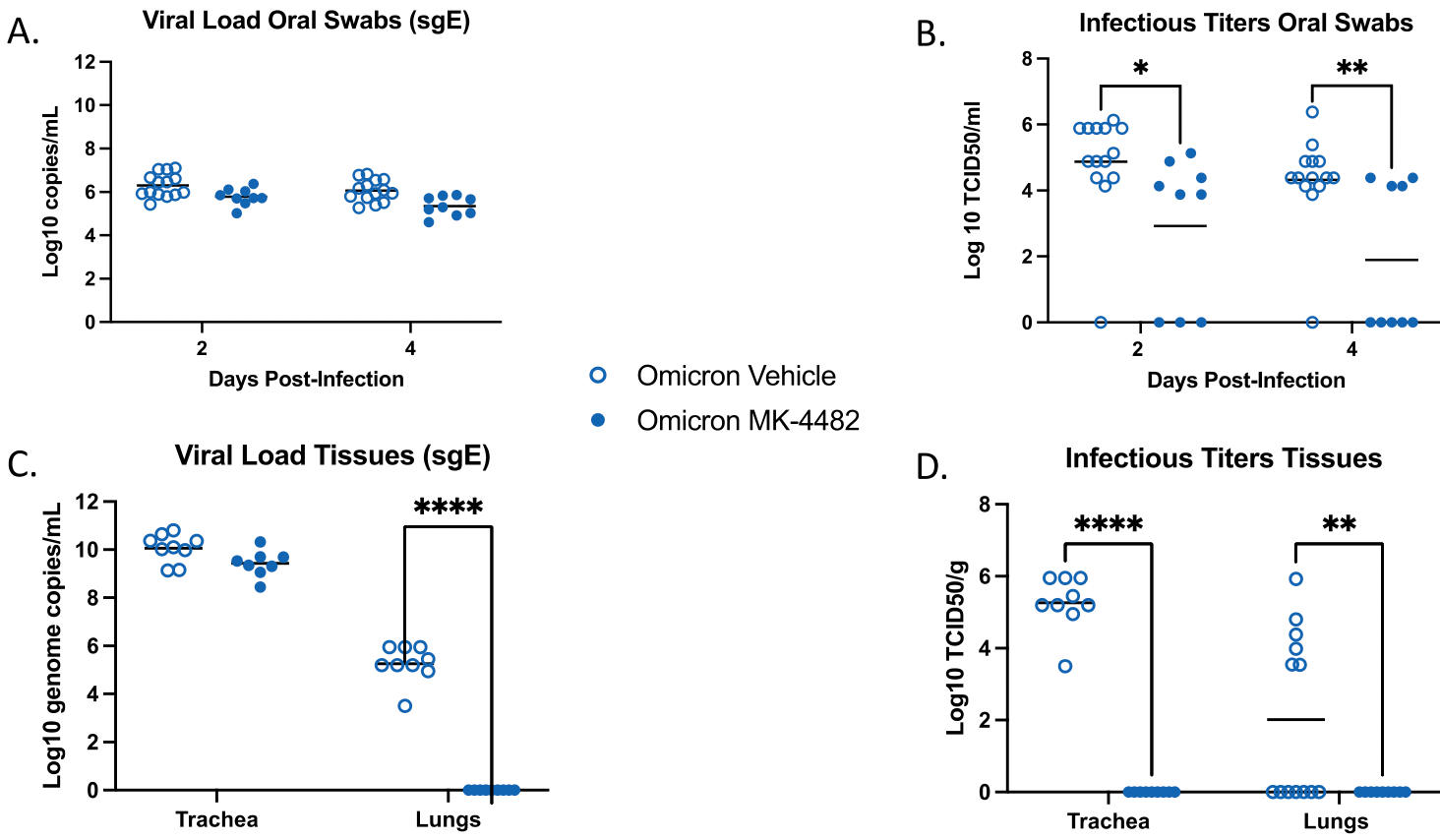
Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model
et al., JCI Insight, doi:10.1172/jci.insight.160108, May 2022
Syrian hamster study showing efficacy of molnupiravir for multiple variants including omicron.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Rosenke et al., 17 May 2022, United Kingdom, peer-reviewed, 11 authors.
Contact: feldmannh@niaid.nih.gov, michael.jarvis@plymouth.ac.uk.
Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model
The recent emergence of the SARS-CoV-2 Omicron variant of concern (VOC) containing a heavily mutated spike protein capable of escaping preexisting immunity identifies a continued need for interventional measures. Molnupiravir (MK-4482), an orally administered nucleoside analog, has demonstrated efficacy against earlier SARS-CoV-2 lineages and was recently approved for SARS-CoV-2 infections in high-risk adults. Here we assessed the efficacy of MK-4482 against the earlier Alpha, Beta and Delta VOCs and Omicron in the hamster COVID-19 model. Omicron replication and associated lung disease in vehicle treated hamsters was reduced compared to the earlier VOCs. MK-4482 treatment inhibited virus replication in the lungs of Alpha, Beta and Delta VOC infected hamsters. Importantly, MK-4482 profoundly inhibited virus replication in the upper and lower respiratory tract of hamsters infected with the Omicron VOC. Consistent with its mutagenic mechanism, MK-4482 treatment had a more pronounced inhibitory effect on infectious titers compared to viral RNA genome load. Histopathologic analysis showed that MK-4482 treatment caused a concomitant reduction in the level of lung disease and viral antigen load in infected hamsters across all VOCs examined. Together, our data indicate the potential of MK-4482 as an effective antiviral against known SARS-CoV-2 VOCs, especially Omicron, and likely future SARS-CoV-2 variants.
References
Abdelnabi, Foo, Jonghe, Maes, Weynand et al., Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model, J Infect Dis
Agostini, Pruijssers, Chappell, Gribble, Lu et al., Small-Molecule Antiviral beta-d-N (4)-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance, J Virol
Bansal, Kumar, Mutational cascade of SARS-CoV-2 leading to evolution and emergence of omicron variant, Virus Res
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Cao, Wang, Jian, Song, Yisimayi, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill
Cox, Wolf, Rk, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Fda, Emergency Use Authorization 108: Letter in response to Merck request that the FDA issue an EUA for the emergency use of molnupiravir for the treatment of mild-tomoderate COVID-19 in certain adults who are at high-risk for
Hansen, Feldmann, Ma, Targeting Ebola virus replication through pharmaceutical intervention, Expert Opin Investig Drugs
Hui, Ho, Cheung, Ng, Ching et al., SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo, Nature
Imai, Iwatsuki-Horimoto, Hatta, Loeber, Halfmann et al., Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development, Proc Natl Acad Sci U S A
Jarvis, Hansen, Rosenke, Haddock, Rollinson et al., Evaluation of drugs for potential repurposing against COVID-19 using a tier-based scoring system, Antivir Ther
Kabinger, Stiller, Schmitzova, Dienemann, Kokic et al., Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Karim, Qa, Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic, Lancet
Lieber, Cox, Sourimant, Wolf, Juergens et al., SARS-CoV-2 variant of concern type and biological sex affect efficacy of molnupiravir in dwarf hamster model of severe COVID-19
Lyngse, Mortensen, Denwood, Christiansen, Moller et al., SARS-CoV-2 Omicron VOC Transmission in Danish Households
Medicines, Preliminary data indicate COVID-19 vaccines remain effective against severe disease and hospitalisation caused by the Omicron variant
Mehta, Bhandari, Raut, Kacimi, Huy, Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation, Front Public Health
Munoz-Fontela, Dowling, Funnell, Gsell, Riveros-Balta et al., Animal models for COVID-19, Nature
Port, Yinda, Owusu, Holbrook, Fischer et al., SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters, Nat Commun
Rosenke, Hansen, Schwarz, Feldmann, Haddock et al., Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model, Nat Commun
Rosenke, Jarvis, Feldmann, Schwarz, Okumura et al., Hydroxychloroquine prophylaxis and treatment is ineffective in macaque and hamster SARS-CoV-2 disease models, JCI Insight
Rosenke, Meade-White, Letko, Clancy, Hansen et al., Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection, Emerg Microbes Infect
Sia, Yan, Chin, Fung, Choy et al., Pathogenesis and transmission of SARS-CoV-2 in golden hamsters, Nature
Suzuki, Yamasoba, Kimura, Wang, Kishimoto et al., Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant, Nature
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant, N Engl J Med
Toots, Yoon, Cox, Hart, Sticher et al., Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia, Sci Transl Med
Trimpert, Vladimirova, Dietert, Abdelgawad, Kunec et al., The Roborovski Dwarf Hamster Is A Highly Susceptible Model for a Rapid and Fatal Course of SARS-CoV-2 Infection, Cell Rep
Urakova, Kuznetsova, Crossman, Sokratian, Guthrie et al., Beta-d-N (4)-Hydroxycytidine Is a Potent Anti-alphavirus Compound That Induces a High Level of Mutations in the Viral Genome, J Virol
Who, Tracking SARS-CoV-2 variants
DOI record:
{
"DOI": "10.1172/jci.insight.160108",
"ISSN": [
"2379-3708"
],
"URL": "http://dx.doi.org/10.1172/jci.insight.160108",
"alternative-id": [
"10.1172/jci.insight.160108"
],
"author": [
{
"affiliation": [],
"family": "Rosenke",
"given": "Kyle",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7779-3059",
"affiliation": [],
"authenticated-orcid": false,
"family": "Okumura",
"given": "Atsushi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6657-7588",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lewis",
"given": "Matthew C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feldmann",
"given": "Friederike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meade-White",
"given": "Kimberly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bohler",
"given": "William F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffin",
"given": "Amanda J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3779-7423",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rosenke",
"given": "Rebecca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8907-8821",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shaia",
"given": "Carl",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0124-4061",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jarvis",
"given": "Michael A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9448-8227",
"affiliation": [],
"authenticated-orcid": false,
"family": "Feldmann",
"given": "Heinz",
"sequence": "additional"
}
],
"container-title": "JCI Insight",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T17:17:42Z",
"timestamp": 1652807862000
},
"deposited": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T17:17:44Z",
"timestamp": 1652807864000
},
"funder": [
{
"award": [
"COVID CAN"
],
"name": "NIAID, NIH"
}
],
"indexed": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T17:42:12Z",
"timestamp": 1652809332206
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5,
17
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T00:00:00Z",
"timestamp": 1652745600000
}
}
],
"link": [
{
"URL": "http://insight.jci.org/articles/view/160108/files/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "232",
"original-title": [],
"prefix": "10.1172",
"published": {
"date-parts": [
[
2022,
5,
17
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
17
]
]
},
"publisher": "American Society for Clinical Investigation",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://insight.jci.org/articles/view/160108"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model",
"type": "journal-article"
}