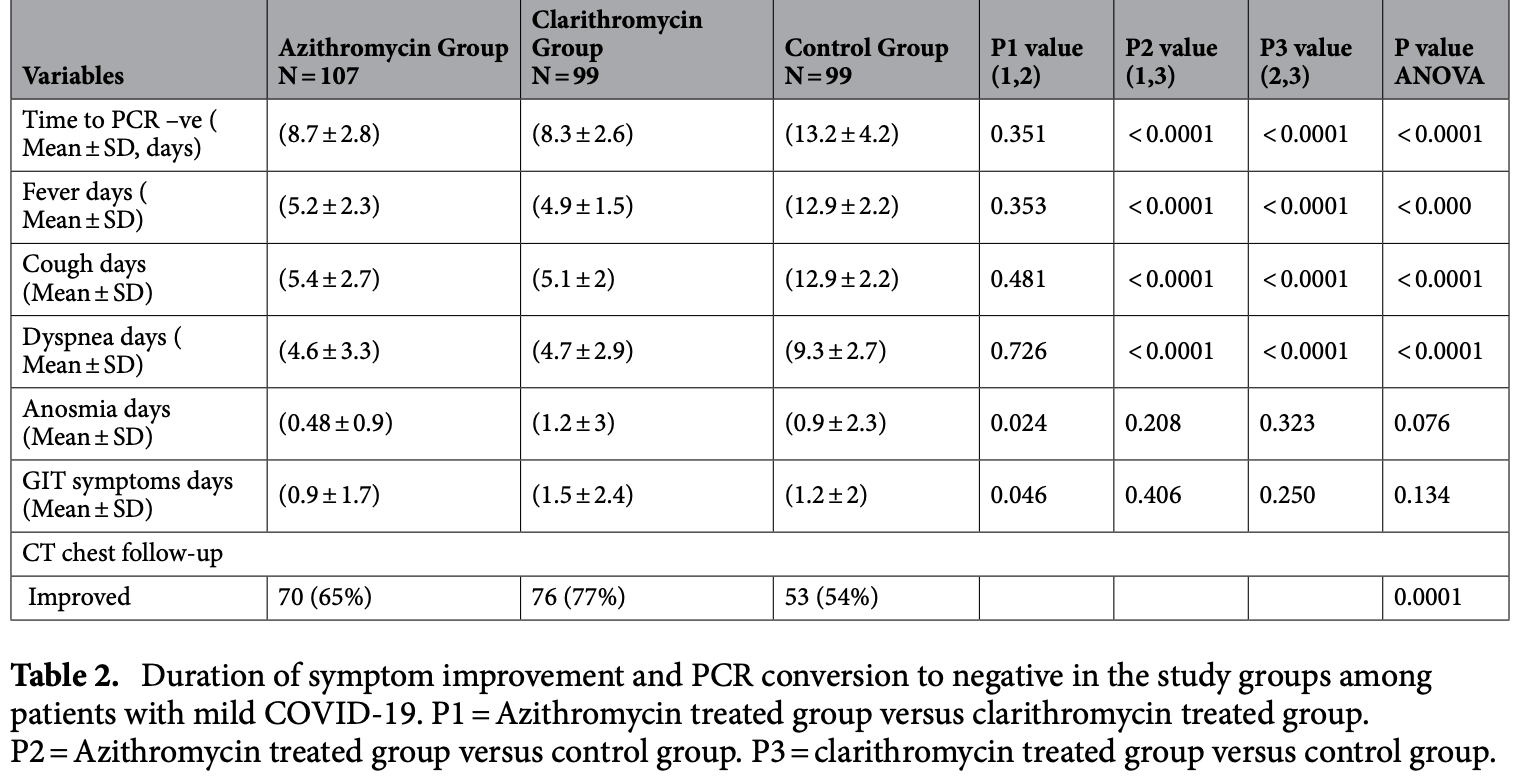
Therapeutic efficacy of macrolides in management of patients with mild COVID-19
et al., Scientific Reports, doi:10.1038/s41598-021-95900-z, NCT04622891, Aug 2021
RCT 305 mild COVID-19 patients showing improved recovery and viral clearance with both azithromycin and clarithromycin.
|
risk of no improvement, 25.6% lower, RR 0.74, p = 0.09, treatment 37 of 107 (34.6%), control 46 of 99 (46.5%), NNT 8.4.
|
|
time to viral-, 34.1% lower, relative time 0.66, p < 0.001, treatment mean 8.7 (±2.8) n=107, control mean 13.2 (±4.2) n=99.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rashad et al., 11 Aug 2021, Randomized Controlled Trial, Egypt, peer-reviewed, 10 authors, study period May 2020 - September 2020, trial NCT04622891 (history).
Contact: mohammedhosnyhassaan@yahoo.com.
Therapeutic efficacy of macrolides in management of patients with mild COVID-19
Scientific Reports, doi:10.1038/s41598-021-95900-z
Evidence on the efficacy of adding macrolides (azithromycin or clarithromycin) to the treatment regimen for COVID-19 is limited. We testify whether adding azithromycin or clarithromycin to a standard of care regimen was superior to standard of supportive care alone in patients with mild COVID-19.This randomized trial included three groups of patients with COVID-19. The azithromycin group included, 107 patients who received azithromycin 500 mg/24 h for 7 days, the clarithromycin group included 99 patients who received clarithromycin 500 /12 h for 7 days, and the control group included 99 patients who received standard care only. All three groups received only symptomatic treatment for control of fever and cough .Clinical and biochemical evaluations of the study participants including assessment of the symptoms duration, real-time reverse transcriptionpolymerase chain reaction (rRT-PCR), C-reactive protein (CRP), serum ferritin, D-dimer, complete blood count (CBC), in addition to non-contrast chest computed tomography (CT), were performed. The overall results revealed significant early improvement of symptoms (fever, dyspnea and cough) in patients treated with either azithromycin or clarithromycin compared to control group, also there was significant early conversion of SARS-CoV-2 PCR to negative in patients treated with either azithromycin or clarithromycin compared to control group (p < 0.05 for all).There was no significant difference in time to improvement of fever, cough, dyspnea, anosmia, gastrointestinal tract "GIT" symptoms and time to PCR negative conversion between patients treated with azithromycin compared to patients treated with clarithromycin (p > 0.05 for all). Follow up chest CT done after 2 weeks of start of treatment showed significant improvement in patients treated with either azithromycin or clarithromycin compared to control group (p < 0.05 for all).Adding Clarithromycin or azithromycin to the therapeutic protocols for COVID-19 could be beneficial for early control of fever and early PCR negative conversion in Mild COVID-19. Trial registration: (NCT04622891) www. Clini calTr ials. gov retrospectively registered (November 10, 2020). SARS-CoV-2 infection is major global health emergency with many countries still experiencing an increase in cases and related fatalities 1,2 . As of December 30, 2020, Egypt has reported 135,333 cases of COVID-19 and 7,574 deaths. Most cases were asymptomatic or had mild to moderate symptoms as dyspnea, fever, dry cough, sore throat, or malaise 3-6 . Mild cases pass unnoticed but they can transmit the infection and increase the disease burden. Therapeutic strategies vary between using newly developed medications and repurposing existing medications for COVID-19. Drugs as remdesivir, lopinavir/ritonavir; (anti HIV drugs), chloroquine and hydroxychloroquine (antimalarial drugs) were recommended in COVID-19 treatment due to their antiviral activity [4] [5] [6] . Other drugs..
Author contributions All authors contributed equally to this work and approved the final version of the manuscript.
Competing interests The authors declare no competing interests.
References
Abdelmaksoud, Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: Possible role of zinc, Biol. Trace Elem. Res, doi:10.1007/s12011-020-02546-5
Aly, Indicators of critical illness and predictors of mortality in COVID-19 patients, Infect Drug Resist
Amodio, Vitale, Cimino, Casuccio, Tramuto, Outbreak of novel coronavirus (SARS-Cov-2): First evidences from International Scientific Literature and Pending Questions, Healthcare (Basel)
Amsden, Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions?, J Antimicrob Chemother [Research Support, Non-U.S. Gov'tReview
Atluri, Manchikanti, Hirsch, Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: The case for compassionate use, Pain Phys
Ballow, Amsden, Azithromycin: the first azalide antibiotic, Ann. Pharmacother
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N Engl J Med
Colson, Rolain, Lagier, Brouqui, Raoult, Chloroquine and hydroxychloroquine as available weapons to fight COVID-19, Int. J. Antimicrob. Agents [EditorialComment
Dai, Chest CT imaging features of typical covert COVID-19 cases, Int. J. Med. Sci
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int. J. Antimicrob. Agents
Gautret, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents [Clinical Trial
Gautret, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents
Ghweil, Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA Quarantine Hospital's Patients, Egypt: A retrospective study, Infect Drug Resist
Hache, Combination of hydroxychloroquine plus azithromycin as potential treatment for COVID-19 patients: safety profile, drug interactions, and management of toxicity, Microb. Drug Resist, doi:10.1089/mdr.2020.0232
Kanoh, Rubin, Mechanisms of action and clinical application of macrolides as immunomodulatory medications, Clin Microbiol Rev [Review
Keyaerts, Vijgen, Maes, Neyts, Van Ranst, In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine, Biochem Biophys Res Commun [Research Support, Non-U.S. Gov't
Kudoh, Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis, Nihon Kyobu Shikkan Gakkai Zasshi [Case Reports
Lal, Mishra, Sahu, CT chest findings in coronavirus disease-19 (COVID-19), J. Formos. Med. Assoc
Lin, Kuo, Hsiao, Lee, Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells, Int. Immunopharmacol
Maeda, Yamada, Nakamura, Maeda, Efficacy of antibiotics against influenza-like illness in an influenza epidemic, Pediatr. Int. [Clinical Trial Randomized Controlled Trial
Martinez, Compounds with therapeutic potential against novel respiratory 2019 coronavirus, Antimicrob. Agents Chemother
Mishra, Designing of cytotoxic and helper T cell epitope map provides insights into the highly contagious nature of the pandemic novel coronavirus SARS-CoV-2, R Soc. Open Sci
Miyamoto, Hasegawa, Sriwilaijaroen, Clarithromycin inhibits progeny virus production from human influenza virusinfected host cells, Biol Pharm Bull [Research Support, Non-U.S. Gov't
Murphy, Azithromycin alters macrophage phenotype, J. Antimicrob. Chemother
Poachanukoon, Koontongkaew, Monthanapisut, Pattanacharoenchai, Macrolides attenuate phorbol ester-induced tumor necrosis factor-α and mucin production from human airway epithelial cells, Pharmacology
Poddighe, Aljofan, Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond, Antivir. Chem. Chemother, doi:10.1177/2040206620961712
Rosenberg, Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA
Rosenberg, Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA [Multicenter Study Observational Study
Sahu, Mishra, Raturi, Lal, Current perspectives of convalescent plasma therapy in COVID-19, Acta Biomed
Sermo, Largest statistically significant study by 6,200 multi-country physicians on COVID-19 uncovers treatment patterns and puts pandemic in context
Stellari, Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study, Pharmacol. Res. Perspect
Sultana, Azithromycin in COVID-19 patients: Pharmacological mechanism, clinical evidence and prescribing guidelines, Drug Saf
Takizawa, Desaki, Ohtoshi, Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells, Am J Respir Crit Care Med [Research Support, Non-U.S. Gov't
Verdejo, Macrolides for the treatment of COVID-19: a living, systematic review, Medwave
Whitman, Tunkel, Azithromycin and clarithromycin: overview and comparison with erythromycin, Infect. Control Hosp. Epidemiol. [Comparative Study Review
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A [Research Support, Non-U.S. Gov't
Yamaya, Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells, J. Pharmacol. Exp. Ther
Yamaya, Shinya, Hatachi, Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells, J Pharmacol Exp Ther [Research Support, Non-U.S. Gov't, doi:10.1038/s41598-021-95900-zwww.nature.com/scientificreports/
Zarogoulidis, Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases, Eur. J. Clin. Pharmacol. [Review
Zhang, Dai, Jian, Lin, Effects of macrolides on airway microbiome and cytokine of children with bronchiolitis: A systematic review and meta-analysis of randomized controlled trials, Microbiol. Immunol [Meta-Analysis Systematic Review
DOI record:
{
"DOI": "10.1038/s41598-021-95900-z",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-021-95900-z",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Evidence on the efficacy of adding macrolides (azithromycin or clarithromycin) to the treatment regimen for COVID-19 is limited. We testify whether adding azithromycin or clarithromycin to a standard of care regimen was superior to standard of supportive care alone in patients with mild COVID-19.This randomized trial included three groups of patients with COVID-19. The azithromycin group included, 107 patients who received azithromycin 500 mg/24 h for 7 days, the clarithromycin group included 99 patients who received clarithromycin 500 /12 h for 7 days, and the control group included 99 patients who received standard care only. All three groups received only symptomatic treatment for control of fever and cough .Clinical and biochemical evaluations of the study participants including assessment of the symptoms duration, real-time reverse transcription-polymerase chain reaction (rRT-PCR), C-reactive protein (CRP), serum ferritin, D-dimer, complete blood count (CBC), in addition to non-contrast chest computed tomography (CT), were performed. The overall results revealed significant early improvement of symptoms (fever, dyspnea and cough) in patients treated with either azithromycin or clarithromycin compared to control group, also there was significant early conversion of SARS-CoV-2 PCR to negative in patients treated with either azithromycin or clarithromycin compared to control group (<jats:italic>p</jats:italic> < 0.05 for all).There was no significant difference in time to improvement of fever, cough, dyspnea, anosmia, gastrointestinal tract \"GIT\" symptoms and time to PCR negative conversion between patients treated with azithromycin compared to patients treated with clarithromycin (<jats:italic>p</jats:italic> > 0.05 for all). Follow up chest CT done after 2 weeks of start of treatment showed significant improvement in patients treated with either azithromycin or clarithromycin compared to control group (<jats:italic>p</jats:italic> < 0.05 for all).Adding Clarithromycin or azithromycin to the therapeutic protocols for COVID-19 could be beneficial for early control of fever and early PCR negative conversion in Mild COVID-19.</jats:p><jats:p><jats:italic>Trial registration</jats:italic>: (NCT04622891) <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://www.ClinicalTrials.gov\">www.ClinicalTrials.gov</jats:ext-link> retrospectively registered (November 10, 2020).</jats:p>",
"alternative-id": [
"95900"
],
"article-number": "16361",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 February 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "11 August 2021"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Rashad",
"given": "Alaa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nafady",
"given": "Asmaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassan",
"given": "Mohammed H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mansour",
"given": "Haggagy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taya",
"given": "Usama",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bazeed",
"given": "Shamardan Ezzeldin S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aref",
"given": "Zaki F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sayed",
"given": "Mennatallah Ali Abdelrhman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nafady-Hego",
"given": "Hanaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelmaksoud",
"given": "Aida A.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04622891",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
11
]
],
"date-time": "2021-08-11T10:09:03Z",
"timestamp": 1628676543000
},
"deposited": {
"date-parts": [
[
2022,
12,
3
]
],
"date-time": "2022-12-03T12:31:36Z",
"timestamp": 1670070696000
},
"funder": [
{
"name": "South valley University, Faculty of Medicine, Qena83523, Egypt."
}
],
"indexed": {
"date-parts": [
[
2025,
4,
16
]
],
"date-time": "2025-04-16T04:48:25Z",
"timestamp": 1744778905026
},
"is-referenced-by-count": 17,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
8,
11
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
11
]
],
"date-time": "2021-08-11T00:00:00Z",
"timestamp": 1628640000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
11
]
],
"date-time": "2021-08-11T00:00:00Z",
"timestamp": 1628640000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-021-95900-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-021-95900-z",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-021-95900-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
8,
11
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
11
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/healthcare8010051",
"author": "E Amodio",
"doi-asserted-by": "publisher",
"first-page": "51",
"issue": "1",
"journal-title": "Healthcare (Basel)",
"key": "95900_CR1",
"unstructured": "Amodio, E., Vitale, F., Cimino, L., Casuccio, A. & Tramuto, F. Outbreak of novel coronavirus (SARS-Cov-2): First evidences from International Scientific Literature and Pending Questions. Healthcare (Basel) 8(1), 51 (2020).",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1098/rsos.201141",
"author": "S Mishra",
"doi-asserted-by": "publisher",
"first-page": "201141",
"issue": "9",
"journal-title": "R Soc. Open Sci.",
"key": "95900_CR2",
"unstructured": "Mishra, S. Designing of cytotoxic and helper T cell epitope map provides insights into the highly contagious nature of the pandemic novel coronavirus SARS-CoV-2. R Soc. Open Sci. 7(9), 201141 (2020).",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.2147/IDR.S261159",
"author": "MH Aly",
"doi-asserted-by": "publisher",
"first-page": "1995",
"journal-title": "Infect Drug Resist.",
"key": "95900_CR3",
"unstructured": "Aly, M. H. et al. Indicators of critical illness and predictors of mortality in COVID-19 patients. Infect Drug Resist. 13, 1995–2000 (2020).",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105938",
"author": "CA Devaux",
"doi-asserted-by": "publisher",
"first-page": "105938",
"issue": "5",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "95900_CR4",
"unstructured": "Devaux, C. A., Rolain, J. M., Colson, P. & Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. Int. J. Antimicrob. Agents 55(5), 105938 (2020).",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2004.08.085",
"doi-asserted-by": "crossref",
"key": "95900_CR5",
"unstructured": "Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun [Research Support, Non-U.S. Gov't]. 2004;323(1):264–8."
},
{
"DOI": "10.1128/AAC.00399-20",
"author": "MA Martinez",
"doi-asserted-by": "publisher",
"first-page": "e00399",
"issue": "5",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "95900_CR6",
"unstructured": "Martinez, M. A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 64(5), e00399-e420 (2020).",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105932",
"author": "P Colson",
"doi-asserted-by": "publisher",
"first-page": "105932",
"issue": "4",
"journal-title": "Int. J. Antimicrob. Agents [EditorialComment].",
"key": "95900_CR7",
"unstructured": "Colson, P., Rolain, J. M., Lagier, J. C., Brouqui, P. & Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents [EditorialComment]. 55(4), 105932 (2020).",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "crossref",
"key": "95900_CR8",
"unstructured": "Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A [Research Support, Non-U.S. Gov't]. 2020;117(20):10970–5."
},
{
"author": "S Atluri",
"first-page": "E71",
"issue": "2",
"journal-title": "Pain Phys.",
"key": "95900_CR9",
"unstructured": "Atluri, S., Manchikanti, L. & Hirsch, J. A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: The case for compassionate use. Pain Phys. 23(2), E71–E83 (2020).",
"volume": "23",
"year": "2020"
},
{
"author": "KK Sahu",
"first-page": "e2020175",
"issue": "4",
"journal-title": "Acta Biomed.",
"key": "95900_CR10",
"unstructured": "Sahu, K. K., Mishra, A. K., Raturi, M. & Lal, A. Current perspectives of convalescent plasma therapy in COVID-19. Acta Biomed. 91(4), e2020175 (2020).",
"volume": "91",
"year": "2020"
},
{
"author": "ES Rosenberg",
"first-page": "2493",
"issue": "24",
"journal-title": "JAMA [Multicenter Study Observational Study].",
"key": "95900_CR11",
"unstructured": "Rosenberg, E. S. et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA [Multicenter Study Observational Study]. 323(24), 2493–2502 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"author": "P Gautret",
"doi-asserted-by": "publisher",
"first-page": "105949",
"issue": "1",
"journal-title": "Int J Antimicrob Agents [Clinical Trial].",
"key": "95900_CR12",
"unstructured": "Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents [Clinical Trial]. 56(1), 105949 (2020).",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkh519",
"doi-asserted-by": "crossref",
"key": "95900_CR13",
"unstructured": "Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother [Research Support, Non-U.S. Gov'tReview]. 2005;55(1):10–21."
},
{
"DOI": "10.1128/CMR.00078-09",
"author": "S Kanoh",
"doi-asserted-by": "publisher",
"first-page": "590",
"issue": "3",
"journal-title": "Clin Microbiol Rev [Review].",
"key": "95900_CR14",
"unstructured": "Kanoh, S. & Rubin, B. K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev [Review]. 23(3), 590–615 (2010).",
"volume": "23",
"year": "2010"
},
{
"DOI": "10.1007/s00228-011-1161-x",
"author": "P Zarogoulidis",
"doi-asserted-by": "publisher",
"first-page": "479",
"issue": "5",
"journal-title": "Eur. J. Clin. Pharmacol. [Review].",
"key": "95900_CR15",
"unstructured": "Zarogoulidis, P. et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. [Review]. 68(5), 479–503 (2012).",
"volume": "68",
"year": "2012"
},
{
"DOI": "10.1177/2040206620961712",
"author": "D Poddighe",
"doi-asserted-by": "publisher",
"first-page": "204020662096171",
"journal-title": "Antivir. Chem. Chemother.",
"key": "95900_CR16",
"unstructured": "Poddighe, D. & Aljofan, M. Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond. Antivir. Chem. Chemother. 28, 2040206620961712. https://doi.org/10.1177/2040206620961712 (2020).",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1177/106002809202601014",
"author": "CH Ballow",
"doi-asserted-by": "publisher",
"first-page": "1253",
"issue": "10",
"journal-title": "Ann. Pharmacother.",
"key": "95900_CR17",
"unstructured": "Ballow, C. H. & Amsden, G. W. Azithromycin: the first azalide antibiotic. Ann. Pharmacother. 26(10), 1253–1261 (1992).",
"volume": "26",
"year": "1992"
},
{
"DOI": "10.1016/j.intimp.2016.09.012",
"author": "SJ Lin",
"doi-asserted-by": "publisher",
"first-page": "318",
"journal-title": "Int. Immunopharmacol.",
"key": "95900_CR18",
"unstructured": "Lin, S. J., Kuo, M. L., Hsiao, H. S. & Lee, P. T. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int. Immunopharmacol. 40, 318–326 (2016 Nov).",
"volume": "40",
"year": "2016"
},
{
"DOI": "10.1093/jac/dkn007",
"author": "BS Murphy",
"doi-asserted-by": "publisher",
"first-page": "554",
"issue": "3",
"journal-title": "J. Antimicrob. Chemother.",
"key": "95900_CR19",
"unstructured": "Murphy, B. S. et al. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 61(3), 554–560 (2008).",
"volume": "61",
"year": "2008"
},
{
"DOI": "10.1159/000358366",
"author": "O Poachanukoon",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "1–2",
"journal-title": "Pharmacology",
"key": "95900_CR20",
"unstructured": "Poachanukoon, O., Koontongkaew, S., Monthanapisut, P. & Pattanacharoenchai, N. Macrolides attenuate phorbol ester-induced tumor necrosis factor-α and mucin production from human airway epithelial cells. Pharmacology 93(1–2), 92–99 (2014).",
"volume": "93",
"year": "2014"
},
{
"DOI": "10.1124/jpet.109.162149",
"author": "M Yamaya",
"doi-asserted-by": "publisher",
"first-page": "81",
"issue": "1",
"journal-title": "J. Pharmacol. Exp. Ther.",
"key": "95900_CR21",
"unstructured": "Yamaya, M. et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Ther. 333(1), 81–90 (2010).",
"volume": "333",
"year": "2010"
},
{
"DOI": "10.1164/ajrccm.156.1.9612065",
"doi-asserted-by": "crossref",
"key": "95900_CR22",
"unstructured": "Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med [Research Support, Non-U.S. Gov't]. 1997;156(1):266–71."
},
{
"DOI": "10.2307/30147135",
"author": "MS Whitman",
"doi-asserted-by": "publisher",
"first-page": "357",
"issue": "6",
"journal-title": "Infect. Control Hosp. Epidemiol. [Comparative Study Review].",
"key": "95900_CR23",
"unstructured": "Whitman, M. S. & Tunkel, A. R. Azithromycin and clarithromycin: overview and comparison with erythromycin. Infect. Control Hosp. Epidemiol. [Comparative Study Review]. 13(6), 357–368 (1992).",
"volume": "13",
"year": "1992"
},
{
"DOI": "10.1007/s12011-020-02546-5",
"author": "AA Abdelmaksoud",
"doi-asserted-by": "publisher",
"journal-title": "Biol. Trace Elem. Res.",
"key": "95900_CR24",
"unstructured": "Abdelmaksoud, A. A. et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: Possible role of zinc. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-020-02546-5 (2021).",
"year": "2021"
},
{
"DOI": "10.2147/IDR.S263489",
"author": "AA Ghweil",
"doi-asserted-by": "publisher",
"first-page": "2375",
"journal-title": "Infect Drug Resist",
"key": "95900_CR25",
"unstructured": "Ghweil, A. A. et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA Quarantine Hospital’s Patients, Egypt: A retrospective study. Infect Drug Resist 13, 2375–2383 (2020).",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1089/mdr.2020.0232",
"author": "G Hache",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "3",
"journal-title": "Microb. Drug Resist.",
"key": "95900_CR26",
"unstructured": "Hache, G. et al. Combination of hydroxychloroquine plus azithromycin as potential treatment for COVID-19 patients: safety profile, drug interactions, and management of toxicity. Microb. Drug Resist. 27(3), 281–290. https://doi.org/10.1089/mdr.2020.0232 (2021 Mar).",
"volume": "27",
"year": "2021"
},
{
"author": "S Kudoh",
"first-page": "632",
"issue": "6",
"journal-title": "Nihon Kyobu Shikkan Gakkai Zasshi [Case Reports].",
"key": "95900_CR27",
"unstructured": "Kudoh, S. et al. Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi [Case Reports]. 25(6), 632–642 (1987).",
"volume": "25",
"year": "1987"
},
{
"DOI": "10.1111/1348-0421.12726",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "343",
"issue": "9",
"journal-title": "Microbiol. Immunol [Meta-Analysis Systematic Review].",
"key": "95900_CR28",
"unstructured": "Zhang, Y., Dai, J., Jian, H. & Lin, J. Effects of macrolides on airway microbiome and cytokine of children with bronchiolitis: A systematic review and meta-analysis of randomized controlled trials. Microbiol. Immunol [Meta-Analysis Systematic Review]. 63(9), 343–349 (2019).",
"volume": "63",
"year": "2019"
},
{
"author": "S Maeda",
"first-page": "274",
"issue": "3",
"journal-title": "Pediatr. Int. [Clinical Trial Randomized Controlled Trial].",
"key": "95900_CR29",
"unstructured": "Maeda, S., Yamada, Y., Nakamura, H. & Maeda, T. Efficacy of antibiotics against influenza-like illness in an influenza epidemic. Pediatr. Int. [Clinical Trial Randomized Controlled Trial]. 41(3), 274–276 (1999).",
"volume": "41",
"year": "1999"
},
{
"DOI": "10.1124/jpet.109.162149",
"doi-asserted-by": "crossref",
"key": "95900_CR30",
"unstructured": "Yamaya M, Shinya K, Hatachi Y, et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J Pharmacol Exp Ther [Research Support, Non-U.S. Gov't]. 2010 ;333(1):81–90."
},
{
"DOI": "10.1248/bpb.31.217",
"doi-asserted-by": "crossref",
"key": "95900_CR31",
"unstructured": "Miyamoto D, Hasegawa S, Sriwilaijaroen N, et al. Clarithromycin inhibits progeny virus production from human influenza virus-infected host cells. Biol Pharm Bull [Research Support, Non-U.S. Gov't]. 2008;31(2):217–22."
},
{
"DOI": "10.1007/s40264-020-00976-7",
"author": "J Sultana",
"doi-asserted-by": "publisher",
"first-page": "691",
"issue": "8",
"journal-title": "Drug Saf.",
"key": "95900_CR32",
"unstructured": "Sultana, J. et al. Azithromycin in COVID-19 patients: Pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 43(8), 691–698 (2020).",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1002/prp2.58",
"author": "FF Stellari",
"doi-asserted-by": "publisher",
"first-page": "00058",
"issue": "5",
"journal-title": "Pharmacol. Res. Perspect.",
"key": "95900_CR33",
"unstructured": "Stellari, F. F. et al. Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study. Pharmacol. Res. Perspect. 2(5), 00058 (2014).",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1001/jama.2020.8630",
"author": "ES Rosenberg",
"doi-asserted-by": "publisher",
"first-page": "2493",
"issue": "24",
"journal-title": "JAMA",
"key": "95900_CR34",
"unstructured": "Rosenberg, E. S. et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 323(24), 2493–2502 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"author": "P Gautret",
"doi-asserted-by": "publisher",
"first-page": "105949",
"issue": "1",
"journal-title": "Int. J. Antimicrob. Agents.",
"key": "95900_CR35",
"unstructured": "Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 56(1), 105949 (2020).",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.5867/medwave.2020.11.8073",
"author": "C Verdejo",
"doi-asserted-by": "publisher",
"first-page": "e8074",
"issue": "11",
"journal-title": "Medwave.",
"key": "95900_CR36",
"unstructured": "Verdejo, C. et al. Macrolides for the treatment of COVID-19: a living, systematic review. Medwave. 20(11), e8074 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2019014",
"doi-asserted-by": "crossref",
"key": "95900_CR37",
"unstructured": "Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052."
},
{
"DOI": "10.7150/ijms.48614",
"author": "M Dai",
"doi-asserted-by": "publisher",
"first-page": "2128",
"issue": "10",
"journal-title": "Int. J. Med. Sci.",
"key": "95900_CR38",
"unstructured": "Dai, M. et al. Chest CT imaging features of typical covert COVID-19 cases. Int. J. Med. Sci. 18(10), 2128–2136 (2021).",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.jfma.2020.03.010",
"author": "A Lal",
"doi-asserted-by": "publisher",
"first-page": "1000",
"issue": "5",
"journal-title": "J. Formos. Med. Assoc.",
"key": "95900_CR39",
"unstructured": "Lal, A., Mishra, A. K. & Sahu, K. K. CT chest findings in coronavirus disease-19 (COVID-19). J. Formos. Med. Assoc. 119(5), 1000–1001 (2020).",
"volume": "119",
"year": "2020"
},
{
"key": "95900_CR40",
"unstructured": "Sermo. Largest statistically significant study by 6,200 multi-country physicians on COVID-19 uncovers treatment patterns and puts pandemic in context. April 2, 2020. https://www.sermo.com/press-releases/largest-statistically-significant-study-by-6200-multi-country-physicians-on-covid-19-uncovers-treatment-patterns-and-puts-pandemic-in-context/ (accessed April 28, 2020)."
}
],
"reference-count": 40,
"references-count": 40,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-181996/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-021-95900-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Therapeutic efficacy of macrolides in management of patients with mild COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "11"
}