Safety and Efficacy of Favipiravir for the management of COVID-19 Patients: A Randomized Control Trial
et al., Clinical Infection in Practice, doi:10.1016/j.clinpr.2022.100145, NCT04402203, May 2022
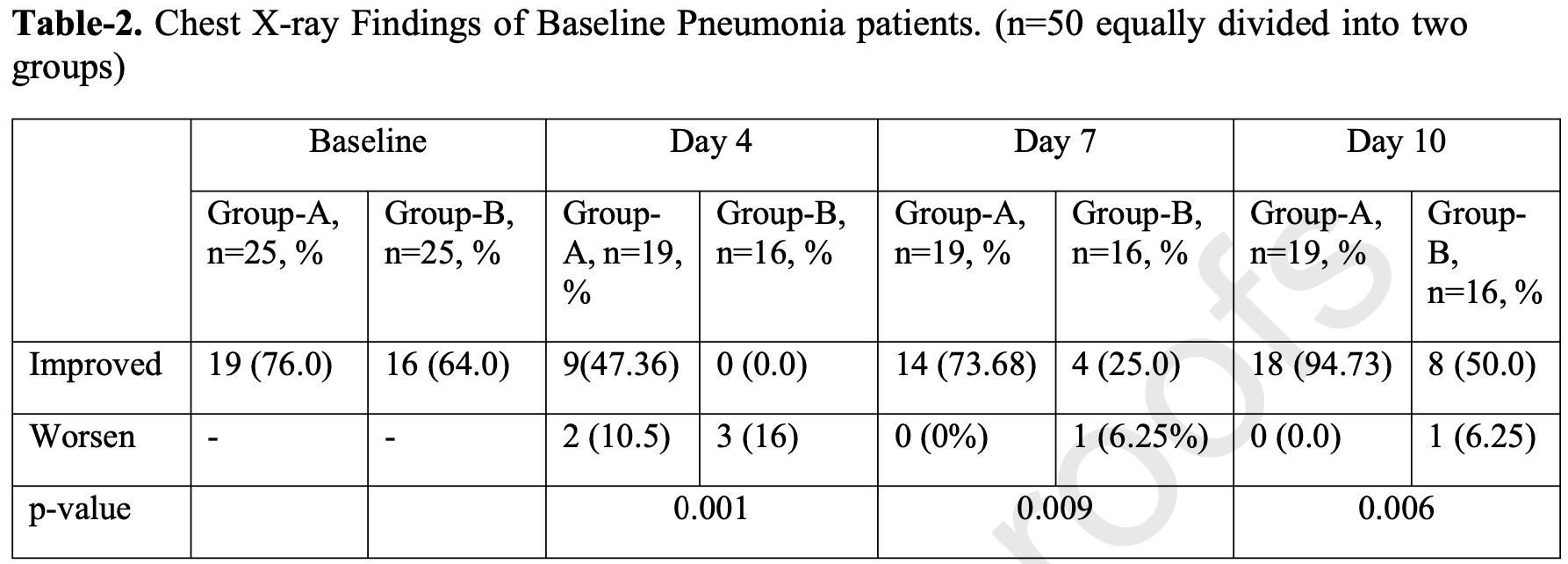
RCT hospitalized patients in Bangladesh, showing faster recovery and viral clearance with favipiravir treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of no chest x-ray improvement, 89.5% lower, RR 0.11, p = 0.005, treatment 1 of 19 (5.3%), control 8 of 16 (50.0%), NNT 2.2, day 10.
|
|
risk of no chest x-ray improvement, 64.9% lower, RR 0.35, p = 0.007, treatment 5 of 19 (26.3%), control 12 of 16 (75.0%), NNT 2.1, day 7.
|
|
risk of no chest x-ray improvement, 47.4% lower, RR 0.53, p = 0.001, treatment 10 of 19 (52.6%), control 16 of 16 (100.0%), NNT 2.1, day 4.
|
|
risk of no viral clearance, 91.7% lower, RR 0.08, p < 0.001, treatment 1 of 25 (4.0%), control 12 of 25 (48.0%), NNT 2.3, day 10.
|
|
risk of no viral clearance, 62.5% lower, RR 0.38, p = 0.010, treatment 6 of 25 (24.0%), control 16 of 25 (64.0%), NNT 2.5, day 7.
|
|
risk of no viral clearance, 48.0% lower, RR 0.52, p < 0.001, treatment 13 of 25 (52.0%), control 25 of 25 (100.0%), NNT 2.1, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Rahman et al., 13 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Bangladesh, peer-reviewed, mean age 37.8, 10 authors, study period May 2020 - July 2020, trial NCT04402203 (history).
Contact: smarahman@du.ac.bd.
Safety and efficacy of favipiravir for the management of COVID-19 patients: A preliminary randomized control trial
Clinical Infection in Practice, doi:10.1016/j.clinpr.2022.100145
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Table-5: Assessment of Adverse effects of patients of both groups 4%) 0 (0%) 0 (0%) 1 (4%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) Jaundice 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) Skin rash 1 (4%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) Liver damage 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 2(8%) 0 (0%) 1 (4%) Anemia 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) Vertigo 2 (8%) 2 (8%) 2 (8%) 2 (8%) 0 (0%) 2 (8%) 0 (0%) 1 (4%) Anosmia 1 (4%) 0 (0%) 1 (4%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%)
Conflict of Interest: The authors declared that there was no conflict of interest regarding the clinical trial. Funding: Beacon Pharmaceuticals Limited, Dhaka, Bangladesh.
References Author Contributions:
References
Alam, Kamal, Sarkar, Zhou, Rahman et al., Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: position standing in 2021, Front Pharmacol, doi:10.3389/fphar.2021.659577
Cai, Experimental treatment with favipiravir for COVID-19 : An open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Callender, The impact of Pre-existing comorbidities and Therapeutic Interventions on COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.01991
Chen, Favipiravir versus arbidol for COVID-19: A randomized clinical trial, doi:10.1101/2020.03.17.20037432
Cucinotta, Vanelli, WHO declares COVID-19 a pandemic, Acta Biomed
Delang, Favipiravir as a potential countermeasure against neglected and emerging RNA viruses, Antiviral Res
Du, Chen, Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clinical Pharmacology and Therapeutics
Filho, Covid-19 and the UN Sustainable Development Goals: Threat to Solidarity or an Opportunity?, Sustainability, doi:10.3390/su12135343
Furuta, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc. Jpn. Acad. Ser. B Phys. Biol. Sci
Furuta, Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Scientific reports
Hossain, Hridoy, Rahman, Ahmad, Major depressive and Generalized anxiety disorders among university students during the second wave of COVID-19 outbreak in Bnagladesh. Asia Pac, J. Pubic Health
Hossain, Rahman, Repurposing therapeutic agents againsts SARS-CoV-2 infection: most promising and neoteric progress, Expert Rev Antiinfective Therapy, doi:10.1080/14787210.2021.1864327
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Li, Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat Rev Drug Discov
Lu, Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle, J Med Virol, doi:10.1002/jmv.25678
Nicola, The Socio-economic implications of the coronavirus pandemic (COVID-19): A review, Int. J. Surg
Nigata, Favipiravir: a new medication for the ebola virus disease pandemic, Disaster Medicine and Public Health preparedness
Pilkington, A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic?, J. Virus Erad
Roser, Coronavirus Disease (COVID-19)-Statistics and Research
Sleeman, In vitro antiviral activity of favipiravir (T-705 ) against drug-resistant influenza and 2009 a (H1N1) viruses, Antimicrob Agents Chemother
Wang, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019nCoV) in vitro, Cell Res
Weiss, Murdoch, Clinical course and mortality risk of severe COVID-19, Lancet
Zhou, Clinical course and risk factors mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/j.clinpr.2022.100145",
"ISSN": [
"2590-1702"
],
"URL": "http://dx.doi.org/10.1016/j.clinpr.2022.100145",
"alternative-id": [
"S2590170222000139"
],
"article-number": "100145",
"author": [
{
"affiliation": [],
"family": "Rahman",
"given": "S.M. Abdur",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kabir",
"given": "Ahmedul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdullah",
"given": "ABM",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Billal Alam",
"given": "Md",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azad",
"given": "Khan Abul Kalam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Titu Miah",
"given": "Md",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mowla",
"given": "Syed Ghulam Mogni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deb",
"given": "Sudip Ranjan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amin",
"given": "Mohammad Robed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asaduzzaman",
"given": "Muhammad",
"sequence": "additional"
}
],
"container-title": "Clinical Infection in Practice",
"container-title-short": "Clinical Infection in Practice",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T03:11:37Z",
"timestamp": 1652325097000
},
"deposited": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T03:11:54Z",
"timestamp": 1652325114000
},
"indexed": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T03:40:55Z",
"timestamp": 1652326855425
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
22
]
],
"date-time": "2022-04-22T00:00:00Z",
"timestamp": 1650585600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2590170222000139?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2590170222000139?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100145",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1002/jmv.25678",
"article-title": "Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "401",
"issue": "4",
"journal-title": "J Med Virol.",
"key": "10.1016/j.clinpr.2022.100145_b0005",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.clinpr.2022.100145_b0010",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "China. Lancet",
"key": "10.1016/j.clinpr.2022.100145_b0015",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.clinpr.2022.100145_b0020",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30633-4",
"article-title": "Clinical course and mortality risk of severe COVID-19",
"author": "Weiss",
"doi-asserted-by": "crossref",
"first-page": "1014",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.clinpr.2022.100145_b0025",
"volume": "395",
"year": "2020"
},
{
"key": "10.1016/j.clinpr.2022.100145_b0030",
"unstructured": "Worldometer: https://www.worldometers.info/coronavirus/. Accessed 5th April, 2021."
},
{
"article-title": "WHO declares COVID-19 a pandemic",
"author": "Cucinotta",
"first-page": "157",
"issue": "1",
"journal-title": "Acta Biomed",
"key": "10.1016/j.clinpr.2022.100145_b0035",
"volume": "91",
"year": "2020"
},
{
"DOI": "10.1177/10105395211014345",
"article-title": "Major depressive and Generalized anxiety disorders among university students during the second wave of COVID-19 outbreak in Bnagladesh",
"author": "Hossain",
"doi-asserted-by": "crossref",
"first-page": "676",
"issue": "5",
"journal-title": "Asia Pac. J. Pubic Health",
"key": "10.1016/j.clinpr.2022.100145_b0040",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.ijsu.2020.04.018",
"article-title": "The Socio-economic implications of the coronavirus pandemic (COVID-19): A review",
"author": "Nicola",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Int. J. Surg.",
"key": "10.1016/j.clinpr.2022.100145_b0045",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.3390/su12135343",
"article-title": "Covid-19 and the UN Sustainable Development Goals: Threat to Solidarity or an Opportunity?",
"author": "Leal Filho",
"doi-asserted-by": "crossref",
"first-page": "5343",
"issue": "13",
"journal-title": "Sustainability",
"key": "10.1016/j.clinpr.2022.100145_b0050",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2021.1864327",
"article-title": "Repurposing therapeutic agents againsts SARS-CoV-2 infection: most promising and neoteric progress",
"author": "Hossain",
"doi-asserted-by": "crossref",
"first-page": "1009",
"issue": "8",
"journal-title": "Expert Rev Antiinfective Therapy.",
"key": "10.1016/j.clinpr.2022.100145_b0055",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.659577",
"article-title": "Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: position standing in 2021",
"author": "Alam",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol.",
"key": "10.1016/j.clinpr.2022.100145_b0060",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/d41573-020-00016-0",
"article-title": "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "149",
"issue": "3",
"journal-title": "Nat Rev Drug Discov.",
"key": "10.1016/j.clinpr.2022.100145_b0065",
"volume": "19",
"year": "2020"
},
{
"key": "10.1016/j.clinpr.2022.100145_b0070",
"unstructured": "NIH US. National Library of Medicine ClinicalTrial.gov. https://clinicaltrials.gov/ct2/home.Accessed September 5, 2020."
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"issue": "2",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.clinpr.2022.100145_b0075",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1017/dmp.2014.151",
"article-title": "Favipiravir: a new medication for the ebola virus disease pandemic",
"author": "Nagata",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "1",
"journal-title": "Disaster Medicine and Public Health preparedness",
"key": "10.1016/j.clinpr.2022.100145_b0080",
"volume": "9",
"year": "2015"
},
{
"DOI": "10.1016/j.antiviral.2018.03.003",
"article-title": "Favipiravir as a potential countermeasure against neglected and emerging RNA viruses",
"author": "Delang",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.clinpr.2022.100145_b0085",
"volume": "153",
"year": "2018"
},
{
"DOI": "10.1128/AAC.01739-09",
"article-title": "In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 a (H1N1) viruses",
"author": "Sleeman",
"doi-asserted-by": "crossref",
"first-page": "2517",
"issue": "6",
"journal-title": "Antimicrob Agents Chemother.",
"key": "10.1016/j.clinpr.2022.100145_b0090",
"volume": "54",
"year": "2010"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res",
"key": "10.1016/j.clinpr.2022.100145_b0095",
"volume": "30",
"year": "2020"
},
{
"article-title": "Experimental treatment with favipiravir for COVID-19: An open-label control study",
"author": "Cai",
"journal-title": "Engineering (Beijing)",
"key": "10.1016/j.clinpr.2022.100145_b0100",
"year": "2020"
},
{
"key": "10.1016/j.clinpr.2022.100145_b0105",
"unstructured": "Roser M, et.al. Coronavirus Disease (COVID-19)-Statistics and Research. Published online at OurworldData.org. retrieved from https://ourworldindata.org/coronavirus. Accessed 8 Sep 2020."
},
{
"DOI": "10.3389/fimmu.2020.01991",
"article-title": "The impact of Pre-existing comorbidities and Therapeutic Interventions on COVID-19",
"author": "Callender",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.clinpr.2022.100145_b0110",
"volume": "11",
"year": "2020"
},
{
"key": "10.1016/j.clinpr.2022.100145_b0115",
"unstructured": "Pharmaceuticals and Medical Devices Agency, Report on the deliberation results – avigan. Japan; Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, 2011. Available at: www.pmda.go.jp/files/000210319.pdf. Accessed September 20, 2020."
},
{
"key": "10.1016/j.clinpr.2022.100145_b0120",
"unstructured": "COVID-19: Glenmark’s favipiravir shows encouraging results in phase 3 clinical trial. The Indian Express. https://www.newindianexpress.com/nation/2020/jul/23/covid-19-glenmarks-favipiravir-shows-encouraging-results-in-phase-3-clinical-trial-2173500.html. Accessed Septembe 23r, 2020."
},
{
"article-title": "Favipiravir versus arbidol for COVID-19: A randomized clinical trial",
"author": "Chen",
"journal-title": "medRxiv.",
"key": "10.1016/j.clinpr.2022.100145_b0125",
"year": "2020"
},
{
"key": "10.1016/j.clinpr.2022.100145_b0130",
"unstructured": "Trial site news. Russia ministry of health approves avifavir (favipiravir) for COVID-19 patients—cuts duration of illness by over 50 %. https://www.trialsitenews.com/russia-ministry-of-health-approves-avifavir-favipiravir-for-covid-19-patients-cuts-duration-of-illness-by-over-50/. Accessed September 1, 2020)."
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Scientific reports.",
"key": "10.1016/j.clinpr.2022.100145_b0135",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "7",
"journal-title": "Proc. Jpn. Acad. Ser. B Phys. Biol. Sci.",
"key": "10.1016/j.clinpr.2022.100145_b0140",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"article-title": "A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic?",
"author": "Pilkington",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "2",
"journal-title": "J. Virus Erad.",
"key": "10.1016/j.clinpr.2022.100145_b0145",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "242",
"issue": "2",
"journal-title": "Clinical Pharmacology and Therapeutics",
"key": "10.1016/j.clinpr.2022.100145_b0150",
"volume": "108",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2590170222000139"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Safety and Efficacy of Favipiravir for the management of COVID-19 Patients: A Randomized Control Trial",
"type": "journal-article"
}