International Multicenter Study Comparing Cancer to Non-Cancer Patients with COVID-19: Impact of Risk Factors and Treatment Modalities on Survivorship
et al., medRxiv, doi:10.1101/2022.08.25.22279181, Aug 2022
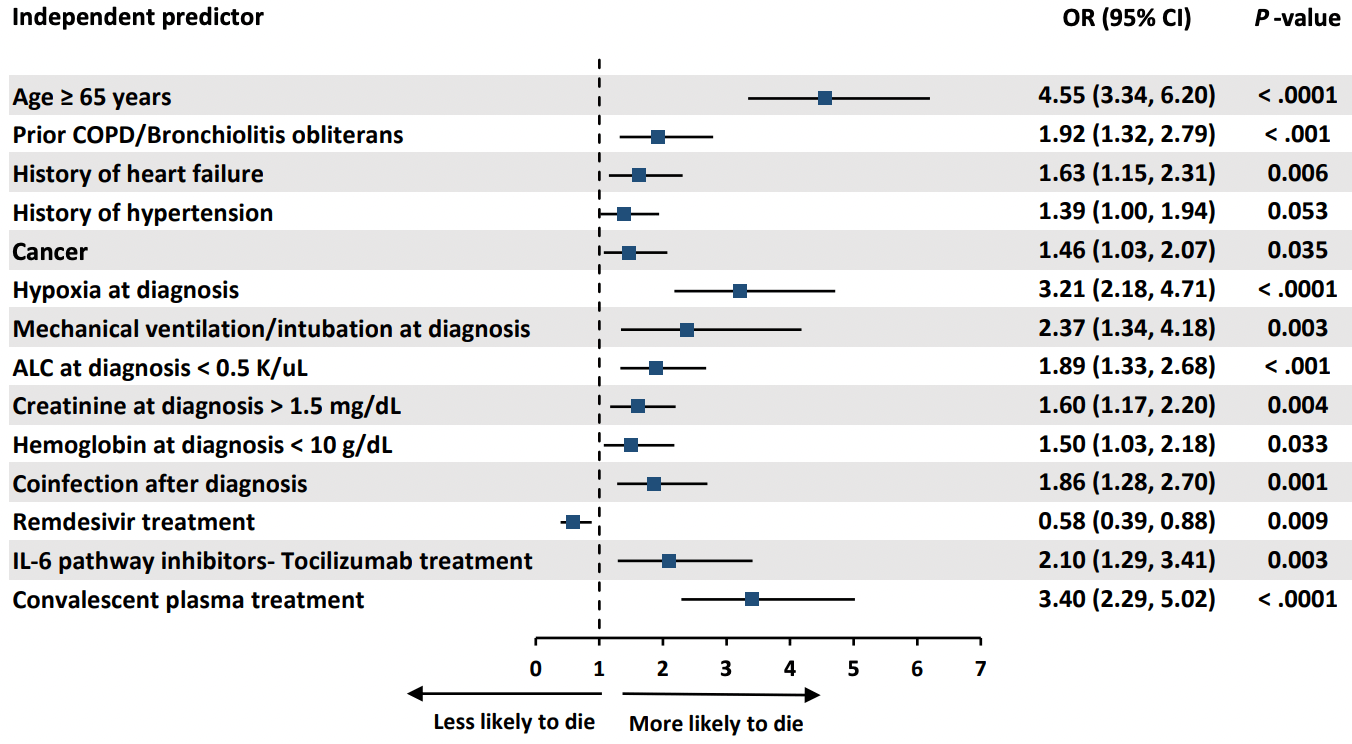
Retrospective 3,966 COVID-19 patients, 1,115 with cancer, showing lower mortality with remdesivir and higher mortality with convalescent plasma.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
Study covers convalescent plasma and remdesivir.
|
risk of death, 42.0% lower, OR 0.58, p = 0.009, adjusted per study, multivariable, day 30, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Raad et al., 26 Aug 2022, retrospective, multiple countries, preprint, 52 authors, study period January 2020 - November 2020.
Contact: achaftari@mdanderson.org, rhachem@mdanderson.org.
International Multicenter Study Comparing Cancer to Non-Cancer Patients with COVID-19: Impact of Risk Factors and Treatment Modalities on Survivorship
doi:10.1101/2022.08.25.22279181
Background: In this international multicenter study we aimed to determine the independent risk factors associated with increased 30-day mortality and the impact of novel treatment modalities in a large group of cancer and non-cancer patients with COVID-19 from multiple countries.
Methods: We retrospectively collected de-identified data on a cohort of cancer and non-cancer patients diagnosed with COVID-19 between January and November 2020, from 16 international centers. Results: We analyzed 3966 COVID-19 confirmed patients, 1115 cancer and 2851 non-cancer patients. Cancer patients were more likely to be pancytopenic, and have a smoking history, pulmonary disorders, hypertension, diabetes mellitus, and corticosteroid use in the preceding two weeks (p≤0.01). In addition, they were more likely to present with higher inflammatory biomarkers (D-dimer, ferritin and procalcitonin), but were less likely to present with clinical symptoms (p≤0.01). By multivariable logistic regression analysis, cancer was an independent risk factor for 30-day mortality (OR 1.46; 95% CI 1.03 to 2.07; p=0.035). Older age (≥65 years) was the strongest predictor of 30-day mortality in all patients (OR 4.55; 95% CI 3.34 to6.20; p< 0.0001). Remdesivir was the only therapeutic agent independently associated with decreased 30day mortality (OR 0.58; CI 0.39-0.88; p=0.009). Among patients on low-flow oxygen at admission, patients who received remdesivir had a lower 30-day mortality rate than those who did not (5.9% vs 17.6%; p=0.03).
Conclusions: Cancer is an independent risk factor for increased 30-day all-cause mortality from COVID-19. Remdesivir, particularly in patients receiving low-flow oxygen, can reduce 30-day all-cause mortality. .
References
Albiges, Foulon, Bayle, Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort, Nat Cancer
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Chavez-Macgregor, Lei, Zhao, Scheet, Giordano, Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients With or Without Cancer, JAMA Oncol
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
He, Chen, Chen, COVID-19 in persons with haematological cancers, Leukemia
Janiaud, Axfors, Schmitt, Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis, JAMA
Klassen, Sj, Johnson, Carter, Wiggins et al., The Effect of convalescent plasma therapy on COVID-19 patient mortality: systematic review and meta-analysis, Mayo Clinic Proceedings
Kucirka, Lauer, Laeyendecker, Boon, Lessler, Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure, Ann Intern Med
Kuderer, Choueiri, Shah, Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study, Lancet
Lai, Chen, Wang, Chen, Wang et al., Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials, J Antimicrob Chemother
Lee, Cazier, Angelis, COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study, Lancet
Libster, Marc, Wappner, Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults, N Engl J Med
Liu, Lin, Baine, Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study, Nat Med
Lunski, Burton, Tawagi, Multivariate mortality analyses in COVID-19: Comparing patients with cancer and patients without cancer in Louisiana
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ
Mehta, Goel, Kabarriti, Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System, Cancer Discov
Mozaffari, Chandak, Zhang, Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort, Clin Infect Dis
Reafc-Twg, Sterne, Murthy, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, JAMA
Recovery, Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Rezagholizadeh, Khiali, Sarbakhsh, Entezari-Maleki, Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis, Eur J Pharmacol
Rivera, Peters, Panagiotou, Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study, Cancer Discov
Robilotti, Babady, Mead, Determinants of COVID-19 disease severity in patients with cancer, Nat Med
Ruthrich, Giessen-Jung, Borgmann, COVID-19 in cancer patients: clinical characteristics and outcome-an analysis of the LEOSS registry, Ann Hematol
Salazar, Christensen, Graviss, Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG, Am J Pathol
Shrestha, Budhathoki, Syed, Rawal, Raut et al., Remdesivir: A potential gamechanger or just a myth? A systematic review and meta-analysis, Life Sci
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: A clinicaltherapeutic staging proposal, J Heart Lung Transplant
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Stc, Pan, Peto, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Tian, Yuan, Xiao, Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study, Lancet Oncol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1101/2022.08.25.22279181",
"URL": "http://dx.doi.org/10.1101/2022.08.25.22279181",
"abstract": "<jats:p>Background: In this international multicenter study we aimed to determine the independent risk factors associated with increased 30-day mortality and the impact of novel treatment modalities in a large group of cancer and non-cancer patients with COVID-19 from multiple countries.\n Methods: We retrospectively collected de-identified data on a cohort of cancer and non-cancer patients diagnosed with COVID-19 between January and November 2020, from 16 international centers.\nResults: We analyzed 3966 COVID-19 confirmed patients, 1115 cancer and 2851 non-cancer patients. Cancer patients were more likely to be pancytopenic, and have a smoking history, pulmonary disorders, hypertension, diabetes mellitus, and corticosteroid use in the preceding two weeks (p≤0.01). In addition, they were more likely to present with higher inflammatory biomarkers (D-dimer, ferritin and procalcitonin), but were less likely to present with clinical symptoms (p≤0.01). By multivariable logistic regression analysis, cancer was an independent risk factor for 30-day mortality (OR 1.46; 95% CI 1.03 to 2.07; p=0.035). Older age (≥65 years) was the strongest predictor of 30-day mortality in all patients (OR 4.55; 95% CI 3.34 to6.20; p< 0.0001). Remdesivir was the only therapeutic agent independently associated with decreased 30-day mortality (OR 0.58; CI 0.39-0.88; p=0.009). Among patients on low-flow oxygen at admission, patients who received remdesivir had a lower 30-day mortality rate than those who did not (5.9% vs 17.6%; p=0.03). \nConclusions: Cancer is an independent risk factor for increased 30-day all-cause mortality from COVID-19. Remdesivir, particularly in patients receiving low-flow oxygen, can reduce 30-day all-cause mortality.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
8,
26
]
]
},
"author": [
{
"affiliation": [],
"family": "Raad",
"given": "Issam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hachem",
"given": "Ray",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nigo",
"given": "Masayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Datoguia",
"given": "Tarcila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dagher",
"given": "Hiba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Ying",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6064-6837",
"affiliation": [],
"authenticated-orcid": false,
"family": "Subbiah",
"given": "Vivek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siddiqui",
"given": "Bilal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayle",
"given": "Arnaud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Somer",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandez-Cruz",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gorak",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhinder",
"given": "Arvinder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nobuyoshi",
"given": "Mori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamerschlak",
"given": "Nelson",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shelanski",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dragovich",
"given": "Tomislav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiat",
"given": "Yee Elise Vong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fakhreddine",
"given": "Suha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanna",
"given": "Pierre Abi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chemaly",
"given": "Roy F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mulanovich",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adachi",
"given": "Javier A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borjan",
"given": "Jovan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khawaja",
"given": "Fareed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Granwehr",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Teny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guevara",
"given": "Eduardo Yepez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres",
"given": "Harrys",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ammakkanavar",
"given": "Natraj Reddy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yibirin",
"given": "Marcel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reyes",
"given": "Cielito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pande",
"given": "Mala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Noman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rojo",
"given": "Raniv",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Shahnoor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deeba",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaftari",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsuo",
"given": "Takahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishikawa",
"given": "Kazuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasegawa",
"given": "Ryo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguado-Noya",
"given": "Ramon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Alvaro Garcia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puchol",
"given": "Cristina Traseira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Dong-Gun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slavin",
"given": "Monica",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0213-5470",
"affiliation": [],
"authenticated-orcid": false,
"family": "Teh",
"given": "Benjamin W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arias",
"given": "Cesar A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kontoyiannis",
"given": "Dimitrios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malek",
"given": "Alexandre E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8097-8452",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chaftari",
"given": "Anne Marie",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Data-Driven Determinants for COVID-19 Oncology Discovery Effort (D3CODE) Team",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T20:15:19Z",
"timestamp": 1661544919000
},
"deposited": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T20:15:19Z",
"timestamp": 1661544919000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T20:41:45Z",
"timestamp": 1661546505711
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
26
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.08.25.22279181",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
8,
26
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
8,
26
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.08.25.22279181"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "International Multicenter Study Comparing Cancer to Non-Cancer Patients with COVID-19: Impact of Risk Factors and Treatment Modalities on Survivorship",
"type": "posted-content"
}