Tenecteplase With Concomitant Anticoagulation for Acute Respiratory Failure in Patients With COVID-19: A Randomized Controlled Trial
et al., Cureus, doi:10.7759/cureus.54298, NCT04505592, Feb 2024
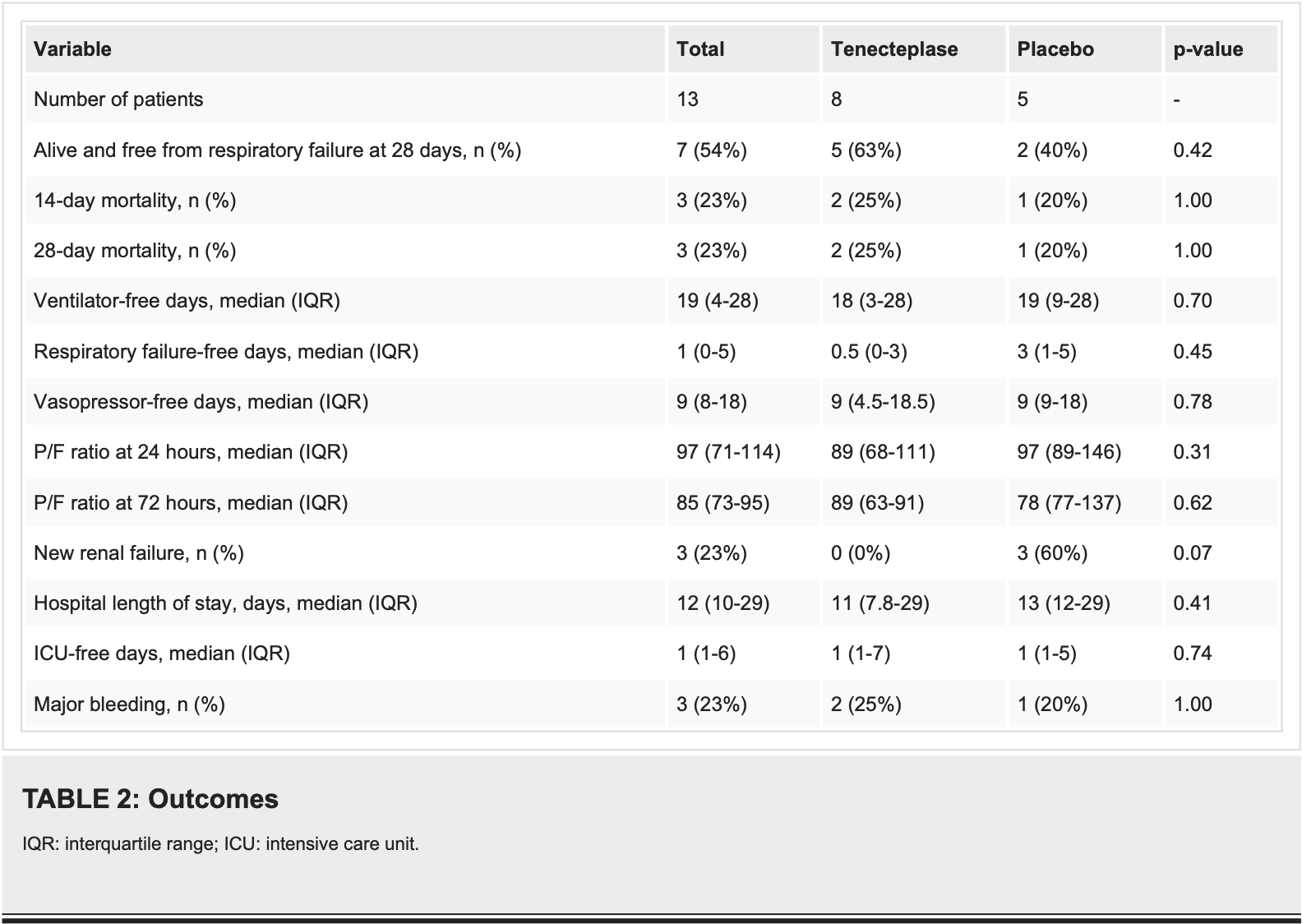
RCT 13 hospitalized COVID-19 patients with acute respiratory failure showing no significant difference in mortality or respiratory outcomes with tenecteplase plus heparin versus placebo plus heparin. At 28 days, 63% of tenecteplase patients were alive and free from respiratory failure compared to 40% in placebo group (p=0.43), with mortality rates of 25% versus 20% respectively (p=1.0). Major bleeding occurred in 25% of tenecteplase patients versus 20% of placebo patients, with no intracranial hemorrhages in either group. The study was severely underpowered due to early termination from poor enrollment (planned 60 patients, enrolled 13), making the results unreliable for drawing meaningful conclusions. Authors hypothesize that tenecteplase's resistance to PAI-1 and ability to be used with concomitant heparin may provide benefits for COVID-19 patients with pulmonary microthrombosis where anticoagulation alone has proven insufficient.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 25.0% higher, RR 1.25, p = 1.00, treatment 2 of 8 (25.0%), control 1 of 5 (20.0%), day 28.
|
|
risk of death, 25.0% higher, RR 1.25, p = 1.00, treatment 2 of 8 (25.0%), control 1 of 5 (20.0%), day 14.
|
|
death or respiratory failure, 37.5% lower, RR 0.62, p = 0.59, treatment 3 of 8 (37.5%), control 3 of 5 (60.0%), NNT 4.4, day 29.
|
|
hospitalization time, 15.4% lower, relative time 0.85, p = 0.82, treatment median 11.0 IQR 21.2 n=8, control median 13.0 IQR 17.0 n=5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Poor et al., 16 Feb 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 68.0, 10 authors, study period September 2020 - March 2021, trial NCT04505592 (history).
Contact: hooman.poor@mountsinai.org.
Tenecteplase is a recombinant tissue plasminogen activator (tPA) and fibrinolytic agent that dissolves blood clots by converting plasminogen to plasmin.
Tenecteplase With Concomitant Anticoagulation for Acute Respiratory Failure in Patients With COVID-19: A Randomized Controlled Trial
Cureus, doi:10.7759/cureus.54298
Background Pulmonary thrombosis and thromboembolism play a significant role in the physiologic derangements seen in COVID-19 acute respiratory failure. The effect of thrombolysis with tenecteplase on patient outcomes is unknown.
Methods We conducted a randomized, controlled, double-blind, phase II trial comparing tenecteplase versus placebo in patients with COVID-19 acute respiratory failure (NCT04505592). Patients with COVID-19 acute respiratory failure were randomized to tenecteplase 0.25 mg/kg or placebo in a 2:1 proportion. Both groups received therapeutic heparin for at least 72 hours.
Results Thirteen patients were included in the trial. Eight patients were randomized to tenecteplase and five were randomized to placebo. At 28 days, 63% (n = 5) of patients assigned to the treatment group were alive and free from respiratory failure compared to 40% (n = 2) in the placebo arm (p = 0.43). Mortality at 28 days was 25% (n = 2) in the treatment arm and 20% (n = 1) in the control arm (p = 1.0). No patients in the treatment arm developed renal failure by 28 days compared to 60% (n = 3) in the placebo arm (p = 0.07). Major bleeding occurred in 25% (n = 2) of the treatment arm and 20% (n = 1) in the placebo arm; however, no patients in either arm experienced intracranial hemorrhage.
Conclusions Tenecteplase with concomitant heparin may improve patient outcomes in patients with COVID-19 respiratory failure. As this study was limited by a small sample size, larger confirmatory studies are needed.
Ethics approval The trial was performed in compliance with the Food and Drug Administration Investigational New Drug regulations (identifier: 150888), was approved by the Institutional Review Board at the Mount Sinai School of Medicine (approval: 20-00894), and was registered with ClinicalTrials.gov (identifier: NCT04505592). Informed consent for trial participation was obtained from the patient, or in the case of incapacitation, the patient's legally authorized representative.
Randomization Patients were randomized to tenecteplase or placebo in a 2:1 fashion using the randomization module
Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board of the Mount Sinai School of Medicine issued approval 20-00894. The trial was performed in compliance with the Food and Drug Administration Investigational New Drug regulations (Identifier: 150888), was approved by the Institutional Review Board of the Mount Sinai School of Medicine (approval 20-00894), and was registered with ClinicalTrials.gov (Identifier: NCT04505592). Informed consent for trial participation was obtained from the patient, or in the case of incapacitation, the patient's legally authorized representative. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: This trial..
References
Ackermann, Verleden, Kuehnel, Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19, N Engl J Med, doi:10.1056/NEJMoa2015432
Barrett, Moore, Moore, Study of alteplase for respiratory failure in SARS-CoV-2 COVID-19: a vanguard multicenter, rapidly adaptive, pragmatic, randomized controlled trial, Chest, doi:10.1016/j.chest.2021.09.024
Chiumello, Busana, Coppola, Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study, Intensive Care Med, doi:10.1007/s00134-020-06281-2
Fahmy, Daas, Salunkhe, Petrey, Cosar et al., Is microthrombosis the main pathology in coronavirus disease 2019 severity?-A systematic review of the postmortem pathologic findings, Crit Care Explor, doi:10.1097/CCE.0000000000000427
Gattinoni, Coppola, Cressoni, Busana, Rossi et al., COVID-19 does not lead to a "typical" acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0817LE
Goligher, Bradbury, Mcverry, Therapeutic anticoagulation with heparin in critically ill patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2103417
Grillet, Behr, Calame, Aubry, Delabrousse, Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography, Radiology, doi:10.1148/radiol.2020201544
Herrmann, Mori, Bates, Suki, Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia, Nat Commun, doi:10.1038/s41467-020-18672-6
Lawler, Goligher, Berger, Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2105911
Meyer, Vicaut, Danays, Fibrinolysis for patients with intermediate-risk pulmonary embolism, N Engl J Med, doi:10.1056/NEJMoa1302097
Oxley, Mocco, Majidi, Large-vessel stroke as a presenting feature of COVID-19 in the young, N Engl J Med, doi:10.1056/NEJMc2009787
Poor, Ventetuolo, Tolbert, COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis, Clin Transl Med, doi:10.1002/ctm2.44
Sholzberg, Tang, Rahhal, Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial, BMJ, doi:10.1136/bmj.n2400
Spiezia, Boscolo, Poletto, COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure, Thromb Haemost, doi:10.1055/s-0040-1710018
Spyropoulos, Goldin, Giannis, Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial, JAMA Intern Med, doi:10.1001/jamainternmed.2021.6203
Wang, Hajizadeh, Moore, Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series, J Thromb Haemost, doi:10.1111/jth.14828
Warach, Dula, Milling, Jr, Tenecteplase thrombolysis for acute ischemic stroke, Stroke, doi:10.1161/STROKEAHA.120.029749
Whyte, Morrow, Mitchell, Chowdary, Mutch, Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19, J Thromb Haemost, doi:10.1111/jth.14872
DOI record:
{
"DOI": "10.7759/cureus.54298",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.54298",
"author": [
{
"affiliation": [],
"family": "Poor",
"given": "Hooman",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yaeger",
"given": "Kurt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deeba",
"given": "Serina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Sydney",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chapman",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xinyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eisenberg",
"given": "Elliot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tolbert",
"given": "Thomas M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shpiner",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mocco",
"given": "J",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
16
]
],
"date-time": "2024-02-16T14:12:36Z",
"timestamp": 1708092756000
},
"deposited": {
"date-parts": [
[
2024,
2,
16
]
],
"date-time": "2024-02-16T14:12:37Z",
"timestamp": 1708092757000
},
"indexed": {
"date-parts": [
[
2024,
2,
17
]
],
"date-time": "2024-02-17T00:30:16Z",
"timestamp": 1708129816455
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2,
16
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/225403-tenecteplase-with-concomitant-anticoagulation-for-acute-respiratory-failure-in-patients-with-covid-19-a-randomized-controlled-trial",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2024,
2,
16
]
]
},
"published-print": {
"date-parts": [
[
2024,
2,
16
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19",
"author": "Ackermann M",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref1",
"unstructured": "Ackermann M, Verleden SE, Kuehnel M, et al.. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020, 383:120-8. 10.1056/NEJMoa2015432",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000427",
"article-title": "Is microthrombosis the main pathology in coronavirus disease 2019 severity?—A systematic review of the postmortem pathologic findings",
"author": "Fahmy OH",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Explor",
"key": "ref2",
"unstructured": "Fahmy OH, Daas FM, Salunkhe V, Petrey JL, Cosar EF, Ramirez J, Akca O. Is microthrombosis the main pathology in coronavirus disease 2019 severity?—A systematic review of the postmortem pathologic findings. Crit Care Explor. 2021, 3:e0427. 10.1097/CCE.0000000000000427",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202003-0817LE",
"article-title": "COVID-19 does not lead to a \"typical\" acute respiratory distress syndrome",
"author": "Gattinoni L",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Crit Care Med",
"key": "ref3",
"unstructured": "Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a \"typical\" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020, 201:1299-300. 10.1164/rccm.202003-0817LE",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06281-2",
"article-title": "Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study",
"author": "Chiumello D",
"doi-asserted-by": "publisher",
"journal-title": "Intensive Care Med",
"key": "ref4",
"unstructured": "Chiumello D, Busana M, Coppola S, et al.. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020, 46:2187-96. 10.1007/s00134-020-06281-2",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-18672-6",
"article-title": "Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia",
"author": "Herrmann J",
"doi-asserted-by": "publisher",
"journal-title": "Nat Commun",
"key": "ref5",
"unstructured": "Herrmann J, Mori V, Bates JH, Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020, 11:4883. 10.1038/s41467-020-18672-6",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1055/s-0040-1710018",
"article-title": "COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure",
"author": "Spiezia L",
"doi-asserted-by": "publisher",
"journal-title": "Thromb Haemost",
"key": "ref6",
"unstructured": "Spiezia L, Boscolo A, Poletto F, et al.. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020, 120:998-1000. 10.1055/s-0040-1710018",
"volume": "120",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020201544",
"article-title": "Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography",
"author": "Grillet F",
"doi-asserted-by": "publisher",
"journal-title": "Radiology",
"key": "ref7",
"unstructured": "Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020, 296:E186-8. 10.1148/radiol.2020201544",
"volume": "296",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2009787",
"article-title": "Large-vessel stroke as a presenting feature of COVID-19 in the young",
"author": "Oxley TJ",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref8",
"unstructured": "Oxley TJ, Mocco J, Majidi S, et al.. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020, 382:e60. 10.1056/NEJMc2009787",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2105911",
"article-title": "Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19",
"author": "Lawler PR",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref9",
"unstructured": "Lawler PR, Goligher EC, Berger JS, et al.. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021, 385:790-802. 10.1056/NEJMoa2105911",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6203",
"article-title": "Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial",
"author": "Spyropoulos AC",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Intern Med",
"key": "ref10",
"unstructured": "Spyropoulos AC, Goldin M, Giannis D, et al.. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021, 181:1612-20. 10.1001/jamainternmed.2021.6203",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2400",
"article-title": "Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial",
"author": "Sholzberg M",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ref11",
"unstructured": "Sholzberg M, Tang GH, Rahhal H, et al.. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021, 375:n2400. 10.1136/bmj.n2400",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103417",
"article-title": "Therapeutic anticoagulation with heparin in critically ill patients with COVID-19",
"author": "Goligher EC",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref12",
"unstructured": "Goligher EC, Bradbury CA, McVerry BJ, et al.. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021, 385:777-89. 10.1056/NEJMoa2103417",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1111/jth.14872",
"article-title": "Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19",
"author": "Whyte CS",
"doi-asserted-by": "publisher",
"journal-title": "J Thromb Haemost",
"key": "ref13",
"unstructured": "Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020, 18:1548-55. 10.1111/jth.14872",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1002/ctm2.44",
"article-title": "COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis",
"author": "Poor HD",
"doi-asserted-by": "publisher",
"journal-title": "Clin Transl Med",
"key": "ref14",
"unstructured": "Poor HD, Ventetuolo CE, Tolbert T, et al.. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Transl Med. 2020, 10:e44. 10.1002/ctm2.44",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1111/jth.14828",
"article-title": "Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series",
"author": "Wang J",
"doi-asserted-by": "publisher",
"journal-title": "J Thromb Haemost",
"key": "ref15",
"unstructured": "Wang J, Hajizadeh N, Moore EE, et al.. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020, 18:1752-5. 10.1111/jth.14828",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2021.09.024",
"article-title": "Study of alteplase for respiratory failure in SARS-CoV-2 COVID-19: a vanguard multicenter, rapidly adaptive, pragmatic, randomized controlled trial",
"author": "Barrett CD",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "ref16",
"unstructured": "Barrett CD, Moore HB, Moore EE, et al.. Study of alteplase for respiratory failure in SARS-CoV-2 COVID-19: a vanguard multicenter, rapidly adaptive, pragmatic, randomized controlled trial. Chest. 2022, 161:710-27. 10.1016/j.chest.2021.09.024",
"volume": "161",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa1302097",
"article-title": "Fibrinolysis for patients with intermediate-risk pulmonary embolism",
"author": "Meyer G",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref17",
"unstructured": "Meyer G, Vicaut E, Danays T, et al.. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014, 370:1402-11. 10.1056/NEJMoa1302097",
"volume": "370",
"year": "2014"
},
{
"DOI": "10.1161/STROKEAHA.120.029749",
"article-title": "Tenecteplase thrombolysis for acute ischemic stroke",
"author": "Warach SJ",
"doi-asserted-by": "publisher",
"journal-title": "Stroke",
"key": "ref18",
"unstructured": "Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020, 51:3440-51. 10.1161/STROKEAHA.120.029749",
"volume": "51",
"year": "2020"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/225403-tenecteplase-with-concomitant-anticoagulation-for-acute-respiratory-failure-in-patients-with-covid-19-a-randomized-controlled-trial"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "Tenecteplase With Concomitant Anticoagulation for Acute Respiratory Failure in Patients With COVID-19: A Randomized Controlled Trial",
"type": "journal-article"
}