Retrospective analysis of the effect of current clinical medications and clinicopathological factors on viral shedding in COVID‑19 patients
et al., Biomedical Reports, doi:10.3892/br.2020.1375, Oct 2020
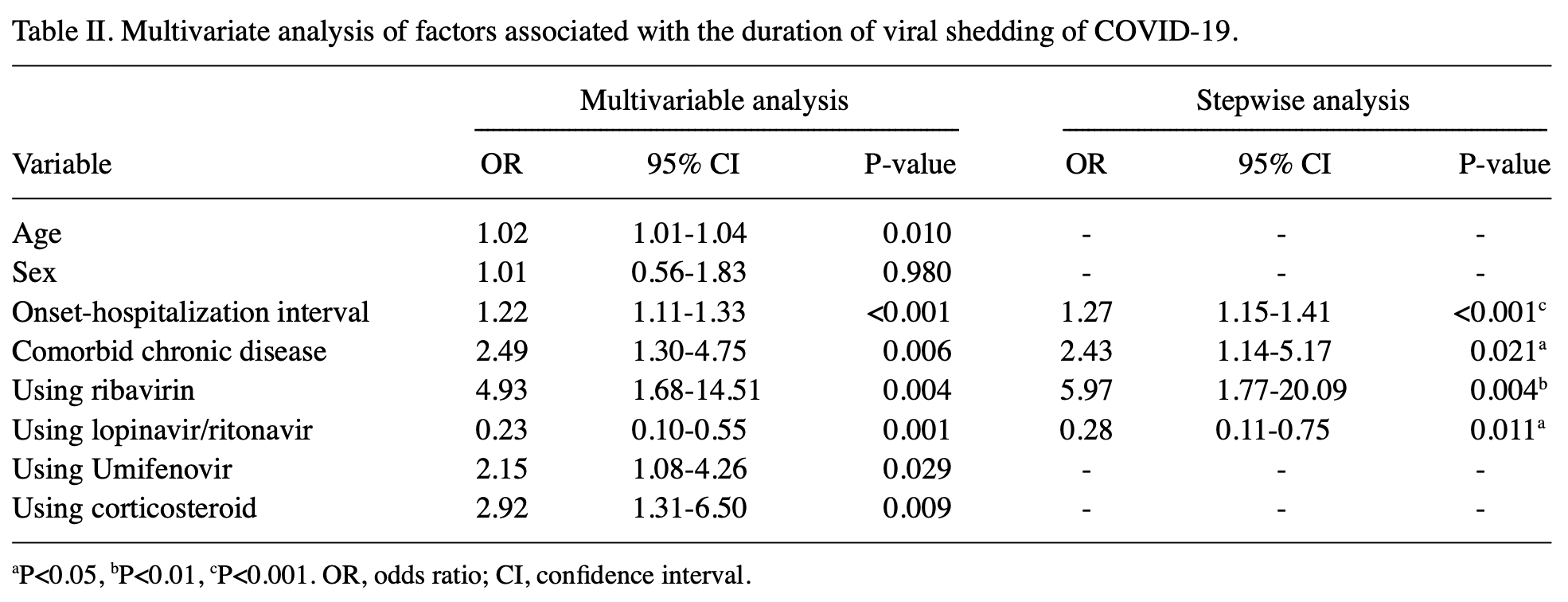
Retrospective 186 hospitalized COVID-19 patients showing faster viral clearance with lopinavir/ritonavir. There may be significant confounding by indication - authors classify cases as non-severe vs severe, but severity is not included in the multivariable model. The higher than expected risk for ribavirin and lower than expected for LPV suggests that unadjusted confounding by indication may significantly affect the results.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
prolonged viral shedding, 45.3% lower, RR 0.55, p = 0.01, treatment 52 of 158 (32.9%), control 19 of 28 (67.9%), NNT 2.9, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pan et al., 20 Oct 2020, retrospective, China, peer-reviewed, median age 46.5, 11 authors, study period 20 January, 2020 - 20 March, 2020.
Contact: panfirstfeng@163.com, goodfreecn@163.com.
Retrospective analysis of the effect of current clinical medications and clinicopathological factors on viral shedding in COVID‑19 patients
Biomedical Reports, doi:10.3892/br.2020.1375
The aim of the present study was to identify the risk factors associated with prolonged shedding in patients with coronavirus disease 2019 , and to evaluate the effects of current clinical and clinicopathological factors on viral shedding in patients. A total of 186 COVID-19 inpatients were enrolled in this multicentre retrospective analysis. Detailed clinical data of each patient were collected, and the factors that affected the duration of viral shedding were retrospectively analysed. The median duration of viral shedding in the 186 COVID-19 patients was 13 days. The median duration of viral shedding was 12 days in non-severe patients, and 17 days in severe patients, and there was a significant difference between the two groups (P<0.001). Multi-factor regression analysis suggested that the onset-hospitalization interval [odds ratio (OR), 1.27; 95% confidence interval (CI), 1.15-1.41; P<0.001] and comorbidity with a chronic disease (OR, 2.43; 95% CI, 1.14-5.17; P=0.021) were independent risk factors for prolonged viral shedding, whereas lopinavir/ritonavir (LPV/r) was an independent protective factor (OR, 0.28; 95% CI, 0.11-0.75; P=0.011). Spearman's rank correlation analysis showed that the onset-drug interval was positively correlated with the duration of viral shedding (r=0.446; P<0.0001). Umifenovir, and low and short courses of glucocorticoids were not associated with prolonged viral shedding. The prolonged viral shedding was the initial causative factor of persistent aggravation of the patient's conditions. The interval between presentation of symptoms and hospitalization as well as complications with a comorbid chronic disease were independent risk factors for prolonged viral shedding. LPV/r shortened the duration of viral shedding, and the smaller the interval between presentation and LPV/r onset was, the faster viral shedding occurred.
Authors' contributions YP contributed to the conception and design of study and the analysis and interpretation of data. QLi contributed to the acquisition, analysis and interpretation of data and manuscript review. XY contributed to the acquisition, analysis and interpretation of data. QLuo contributed to the interpretation of data and manuscript review. TQ, NX, QZhang, XL, XD, QZhao and LS contributed to the acquisition of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate This study was exempt from the need to obtain patient consent due to the retrospective nature of the study, and was approved by the Medical Ethics Committee of Zhengzhou University (approval no. 2020-KY-162).
Patient consent for publication Not applicable.
Competing interests The authors declare that they have no competing interests.
References
Calina, Docea, Petrakis, Egorov, Ishmukhametov et al., Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review), Int J Mol Med
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, N Engl J Med
Chan, Yao, Yeung, Deng, Bao et al., Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset, J Infect Dis
Chu, Cheng, Hung, Wong, Chan et al., Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings, Thorax
Dong, Hu, Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), Drug Discov Ther
Du, Yu, Li, Li, Qin et al., Duration for carrying SARS-CoV-2 in COVID-19 patients, J Infect
Giacomelli, Pagani, Ridolfo, Oreni, Conti et al., Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS-CoV-2 infection: A retrospective cohort study, J Med Virol
Guan, Liang, Zhao, Liang, Chen et al., Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis, Eur Respir J
Karolyi, Pawelka, Mader, Omid, Kelani et al., Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19 patients: Results from a real-life patient cohort, Wien Klin Wochenschr
Lee, Chan, Hui, Ng, Wu et al., Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients, J Clin Virol
Meini, Pagotto, Longo, Vendramin, Pecori et al., Role of Lopinavir/ritonavir in the treatment of Covid-19: A review of current evidence, guideline recommendations, and perspectives, J Clin Med
Nitulescu, Paunescu, Moschos, Petrakis, Nitulescu et al., Comprehensive analysis of drugs to treat SARS-CoV-2 infection: Mechanistic insights into current COVID-19 therapies (Review), Int J Mol Med
Pan, Yu, Du, Li, Li et al., Epidemiological and clinical characteristics of 26 asymptomatic severe acute respiratory syndrome coronavirus 2 carriers, J Infect Dis
Petrakis, Margină, Tsarouhas, Tekos, Stan et al., Obesity-a risk factor for increased COVID-19 prevalence, severity and lethality, Int J Mol Med
Prescott, Rice, Corticosteroids in COVID-19 ARDS: Evidence and hope during the pandemic, JAMA
Sarzi-Puttini, Sirotti, Marotto, Ardizzone, Rizzardini et al., COVID-19, cytokines and immunosuppression: What can we learn from severe acute respiratory syndrome?, Clin Exp Rheumatol
Sheahan, Sims, Leist, Schäfer, Won et al., Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV, Nat Commun
Sibila, Agustí, Torres, Corticosteroids in severe pneumonia, Eur Respir J
Skalny, Rink, Ajsuvakova, Aschner, Gritsenko et al., Zinc and respiratory tract infections: Perspectives for COVID-19 (Review), Int J Mol Med
Sohrabi, Alsafi, Neill, Khan, Kerwan et al., World Health Organization declares global emergency: A review of the 2019 Novel Coronavirus (COVID-19), Int J Surg
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis, JAMA
Tong, Su, Yu, Wu, Chen et al., Ribavirin therapy for severe COVID-19: A retrospective cohort study, Int J Antimicrob Agents
Tsang, Cowling, Fang, Chan, Ip et al., Influenza A virus shedding and infectivity in households, J Infect Dis
Yan, Liu, Zhu, Huang, Dan et al., Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection, Eur Respir J
Young, Ong, Kalimuddin, Low, Tan et al., Epidemiologic features and clinical course of patients infected With SARS-CoV-2 in Singapore, JAMA
Zhang, Wang, Tu, Peng, Huang et al., A comparative study on the time to achieve negative nucleic acid testing and hospital stays between danoprevir and Lopinavir/Ritonavir in the treatment of patients with COVID-19, J Med Virol
Zhao, Hu, Du, Chen, Zhou et al., Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia, Zhonghua Jie He Hu Xi Za Zhi
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
Zhu, Zhang, Li, Yang, Song et al., A novel coronavirus from patients with pneumonia in China, N Engl J Med
Zuo, Liu, Zhong, Zhang, Xu, Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: A retrospective study in two designated hospitals in Anhui, China, J Med Virol
DOI record:
{
"DOI": "10.3892/br.2020.1375",
"ISSN": [
"2049-9434",
"2049-9442"
],
"URL": "http://dx.doi.org/10.3892/br.2020.1375",
"author": [
{
"ORCID": "https://orcid.org/0000-0002-2438-4016",
"affiliation": [
{
"name": "Department of Infectious Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, P.R. China"
}
],
"authenticated-orcid": false,
"family": "Pan",
"given": "Yanfeng",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Li",
"given": "Qingqing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Yu",
"given": "Xue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hepatobiliary and Pancreatic Surgery, Zhengzhou University People's Hospital, Henan Provincial People's Hospital, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Luo",
"given": "Qiankun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hepatobiliary and Pancreatic Surgery, Zhengzhou University People's Hospital, Henan Provincial People's Hospital, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Qin",
"given": "Tao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Zhengzhou Sixth People's Hospital, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Xin",
"given": "Ningbo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Henan Provincial People's Hospital, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Zhang",
"given": "Qian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Shenqiu County People's Hospital, Zhoukou, Henan 466300, P.R. China"
}
],
"family": "Li",
"given": "Xianyang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The People's Hospital of Suzhou New District, Suzhou, Jiangsu 205011, P.R. China"
}
],
"family": "Du",
"given": "Xinwei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Zhengzhou Sixth People's Hospital; 4Department of Infectious Diseases, Henan Provincial People's Hospital, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Zhao",
"given": "Qingxia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, P.R. China"
}
],
"family": "Sun",
"given": "Li",
"sequence": "additional"
}
],
"container-title": "Biomedical Reports",
"container-title-short": "Biomed Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
20
]
],
"date-time": "2020-10-20T10:14:40Z",
"timestamp": 1603188880000
},
"deposited": {
"date-parts": [
[
2020,
10,
20
]
],
"date-time": "2020-10-20T10:14:41Z",
"timestamp": 1603188881000
},
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T19:46:33Z",
"timestamp": 1740167193800,
"version": "3.37.3"
},
"is-referenced-by-count": 1,
"issue": "6",
"issued": {
"date-parts": [
[
2020,
10,
20
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2020,
10,
20
]
]
}
},
"member": "2249",
"original-title": [],
"page": "1-1",
"prefix": "10.3892",
"published": {
"date-parts": [
[
2020,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
20
]
]
},
"publisher": "Spandidos Publications",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.spandidos-publications.com/10.3892/br.2020.1375"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Retrospective analysis of the effect of current clinical medications and clinicopathological factors on viral shedding in COVID‑19 patients",
"type": "journal-article",
"volume": "13"
}