Phase 2, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose Escalation and Proof-of-Concept Study to Evaluate the Safety and Efficacy of Razuprotafib in Hospitalized Subjects With Coronavirus Disease 2019
et al., NCT04511650, RESCUE, NCT04511650, Feb 2021
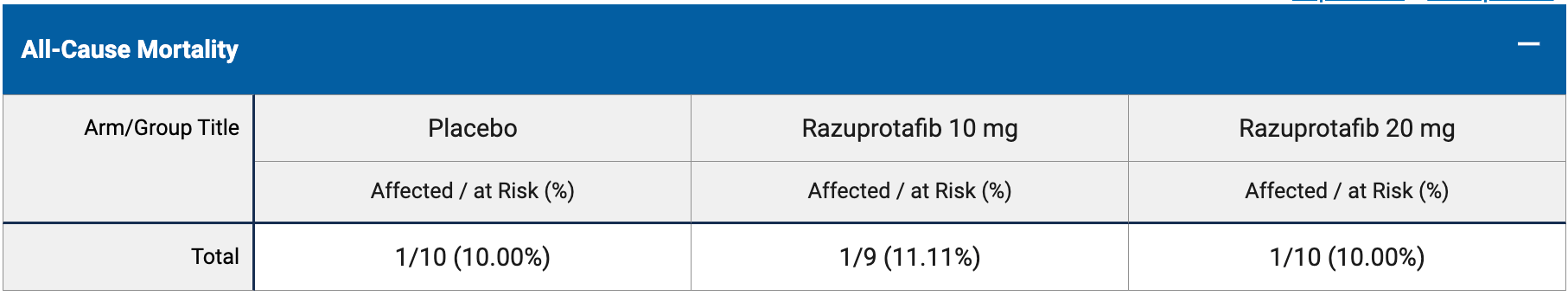
RCT 29 patients showing no significant differences with razuprotafib treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 5.3% higher, RR 1.05, p = 1.00, treatment 2 of 19 (10.5%), control 1 of 10 (10.0%).
|
|
death or respiratory failure, 177.8% higher, RR 2.78, p = 0.37, treatment 5 of 18 (27.8%), control 1 of 10 (10.0%), day 28.
|
|
risk of no hospital discharge, 468.7% higher, RR 5.69, p = 0.28, treatment 3 of 16 (18.8%), control 0 of 9 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Paggiarino et al., 26 Feb 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04511650 (history) (RESCUE).
Contact: dpaggiarino@eyepointpharma.com.
Razuprotafib (AKB-9778) is a small molecule inhibitor of vascular endothelial protein tyrosine phosphatase (VE-PTP) administered via subcutaneous injection. By inhibiting VE-PTP, the drug restores Tie2 receptor signaling, which promotes vascular stability and reduces endothelial permeability.