Razuprotafib (AKB-9778) is a small molecule inhibitor of vascular endothelial protein tyrosine phosphatase (VE-PTP) administered via subcutaneous injection. By inhibiting VE-PTP, the drug restores Tie2 receptor signaling, which promotes vascular stability and reduces endothelial permeability.
Feb 21 |
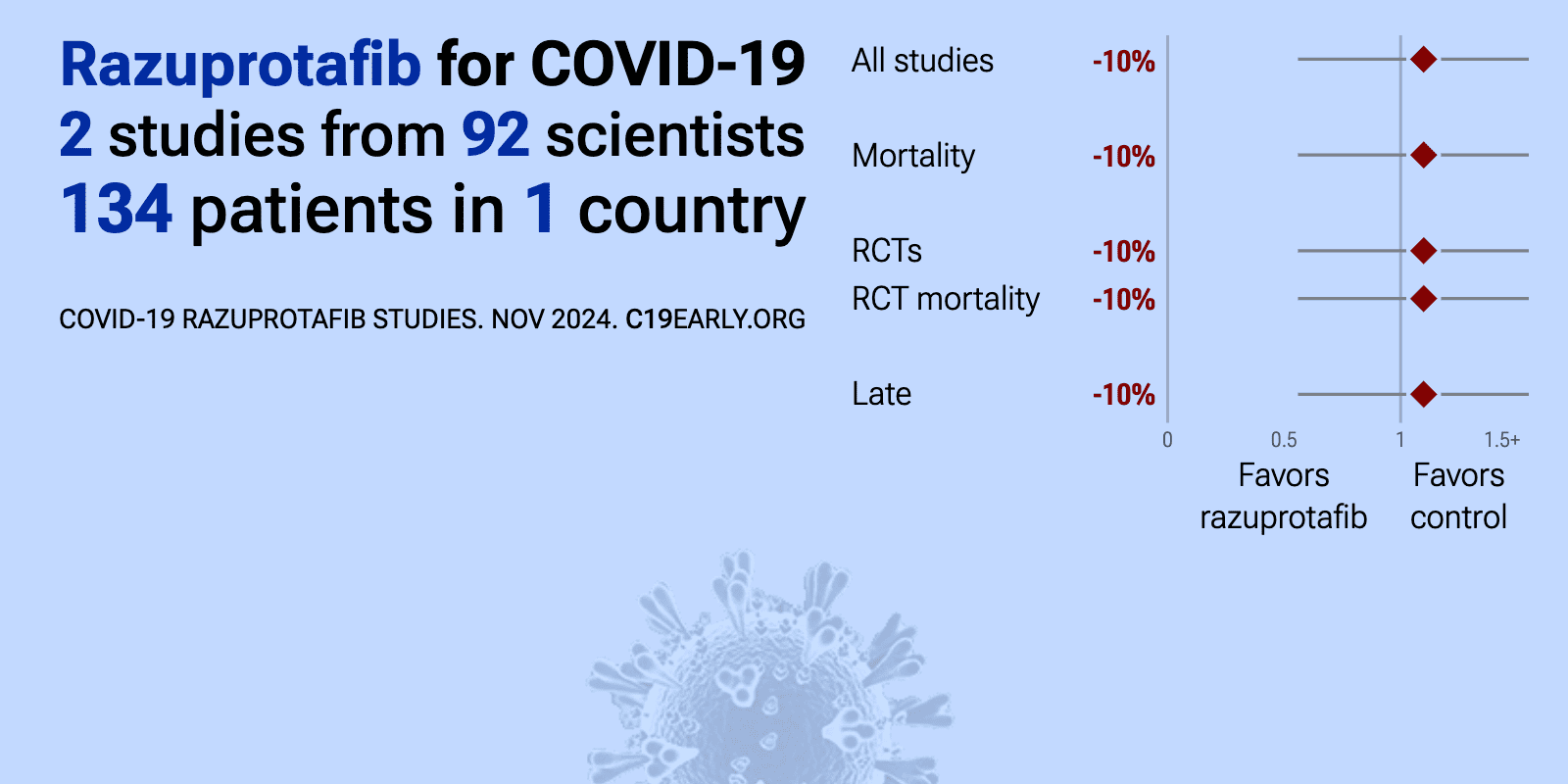
Meta-analysis of razuprotafib studies | |
| Meta-analysis of razuprotafib studies | ||
Mar 3 2023 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.101889 | Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial |
| 10% higher mortality (p=0.81). RCT severe COVID-19 patients showing no significant difference in outcomes with razuprotafib. | ||
Feb 26 2021 |
et al., NCT04511650 | Phase 2, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose Escalation and Proof-of-Concept Study to Evaluate the Safety and Efficacy of Razuprotafib in Hospitalized Subjects With Coronavirus Disease 2019 |
| 178% higher progression (p=0.37) and 469% lower hospital discharge (p=0.28). RCT 29 patients showing no significant differences with razuprotafib treatment. | ||