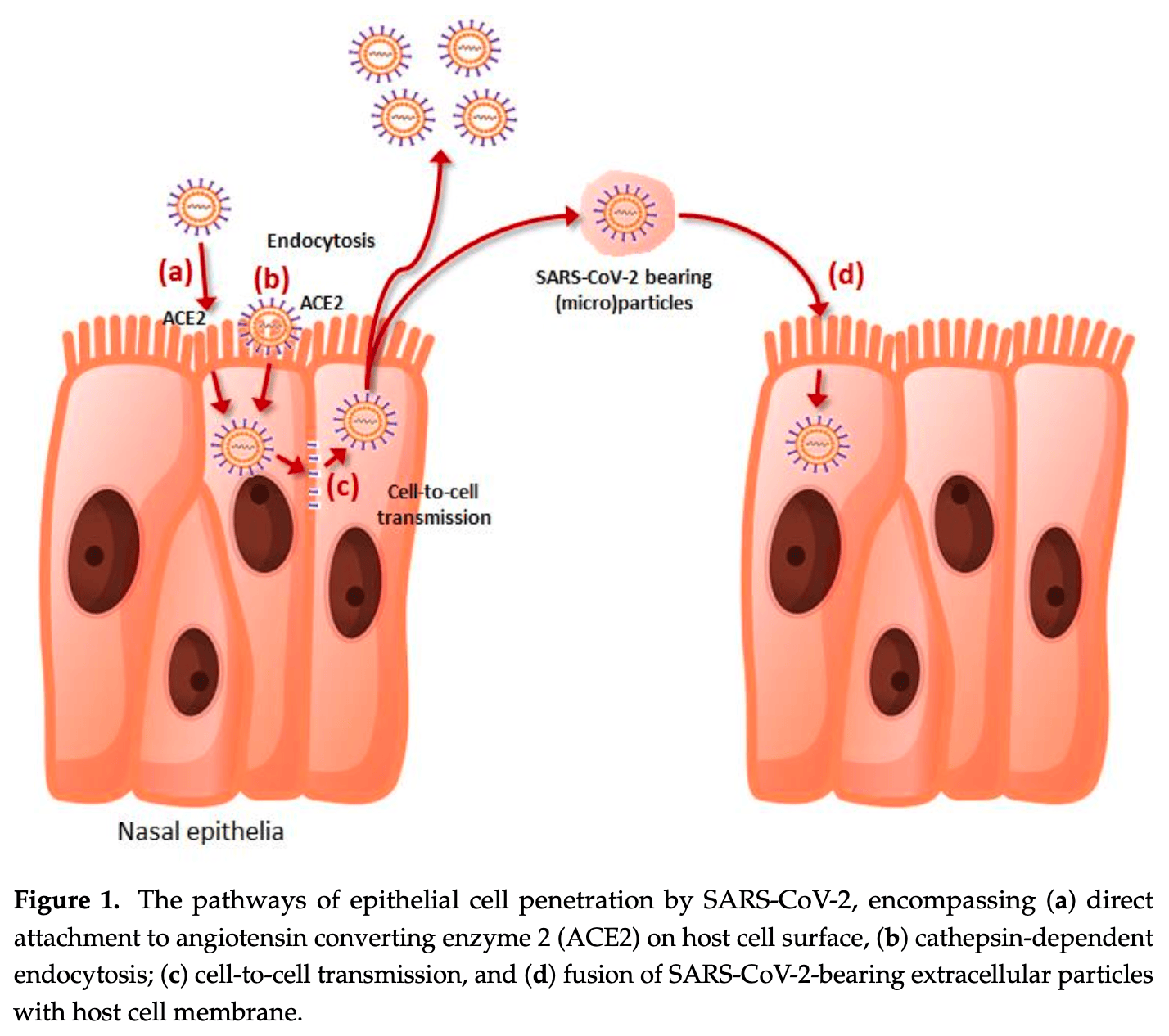
Improving Nasal Protection for Preventing SARS-CoV-2 Infection
et al., Biomedicines, doi:10.3390/biomedicines10112966, Nov 2022
Review of strategies for improving nasal protection to prevent SARS-CoV-2 infection. Authors note the nasal epithelium is the primary entry point for SARS-CoV-2, especially for Omicron variants which replicate efficiently in the upper respiratory tract. While mucosal immunity may provide protection, this immunity has significant limitations: it's absent in SARS-CoV-2 naïve individuals, offers limited protection against new variants, and wanes over time (typically within 100-200 days). Authors explore alternative protective strategies focusing on nasal sprays that create physical barriers or disrupt viral binding to respiratory cells. Several promising compounds have been studied including carrageenan-based formulations, lipopeptide fusion inhibitors, mucoadhesive formulations, monoclonal antibody sprays, hydroxypropyl methylcellulose sprays, astodrimer sodium, and heparin-based formulations.
Review covers iota-carrageenan and astodrimer sodium.
1.
Lefter et al., Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic, Microbiology Research, doi:10.3390/microbiolres15020035.
2.
Chavda et al., Nasal sprays for treating COVID-19: a scientific note, Pharmacological Reports, doi:10.1007/s43440-023-00463-7.
3.
Nocini et al., Improving Nasal Protection for Preventing SARS-CoV-2 Infection, Biomedicines, doi:10.3390/biomedicines10112966.
Nocini et al., 17 Nov 2022, peer-reviewed, 4 authors.
Contact: giuseppe.lippi@univr.it (corresponding author).
Improving Nasal Protection for Preventing SARS-CoV-2 Infection
Biomedicines, doi:10.3390/biomedicines10112966
Airborne pathogens, including SARS-CoV-2, are mainly contracted within the airway pathways, especially in the nasal epithelia, where inhaled air is mostly filtered in resting conditions. Mucosal immunity developing after SARS-CoV-2 infection or vaccination in this part of the body represents one of the most efficient deterrents for preventing viral infection. Nonetheless, the complete lack of such protection in SARS-CoV-2 naïve or seronegative subjects, the limited capacity of neutralizing new and highly mutated lineages, along with the progressive waning of mucosal immunity over time, lead the way to considering alternative strategies for constructing new walls that could stop or entrap the virus at the nasal mucosa surface, which is the area primarily colonized by the new SARS-CoV-2 Omicron sublineages. Among various infection preventive strategies, those based on generating physical barriers within the nose, aimed at impeding host cell penetration (i.e., using compounds with mucoadhesive properties, which act by hindering, entrapping or adsorbing the virus), or those preventing the association of SARS-CoV-2 with its cellular receptors (i.e., administering anti-SARS-CoV-2 neutralizing antibodies or agents that inhibit priming or binding of the spike protein) could be considered appealing perspectives. Provided that these agents are proven safe, comfortable, and compatible with daily life, we suggest prioritizing their usage in subjects at enhanced risk of contagion, during high-risk activities, as well as in patients more likely to develop severe forms of SARS-CoV-2 infection.
References
Altarawneh, Chemaitelly, Ayoub, Hasan, Coyle et al., Protective Effect of Previous SARS-CoV-2 Infection against Omicron BA.4 and BA.5 Subvariants, N. Engl. J. Med, doi:10.1056/NEJMc2209306
Armando, Beythien, Kaiser, Allnoch, Heydemann et al., SARS-CoV-2 Omicron variant causes mild pathology in the upper and lower respiratory tract of hamsters, Nat. Commun, doi:10.1038/s41467-022-31200-y
Bentley, Stanton, Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread, Viruses, doi:10.3390/v13122345
Bovard, Van Der Toorn, Schlage, Constant, Renggli et al., Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochem. Biophys. Rep, doi:10.1016/j.bbrep.2021.101187
Camner, Bakke, Nose or mouth breathing?, Environ. Res, doi:10.1016/0013-9351(80)90042-0
Carpenè, Negrini, Lippi, Favaloro, Montagnana, Heparin: The Journey from Parenteral Agent to Nasal Delivery, Semin. Thromb. Hemost, doi:10.1055/s-0042-1749395
Carvalho, Intranasal COVID-19 vaccine fails to induce mucosal immunity, Nat. Med, doi:10.1038/d41591-022-00106-z
Cucinotta, Vanelli, WHO Declares COVID-19 a Pandemic, Acta Biomed, doi:10.23750/abm.v91i1.9397
De Vries, Schmitz, Bovier, Predella, Khao et al., Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets, Science, doi:10.1126/science.abf4896
Dorabawila, Hoefer, Bauer, Bassett, Lutterloh et al., Risk of Infection and Hospitalization Among Vaccinated and Unvaccinated Children and Adolescents in New York After the Emergence of the Omicron Variant, JAMA, doi:10.1001/jama.2022.7319
Eder, Bermejo-Jambrina, Vlaming, Kaptein, Zaderer et al., Inhalation of Low Molecular Weight Heparins as Prophylaxis against SARS-CoV-2, mBio, doi:10.1128/mbio.02558-22
Ejemel, Li, Hou, Schiller, Tree et al., A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction, Nat. Commun, doi:10.1038/s41467-020-18058-8
Evans, Liu, Role of host factors in SARS-CoV-2 entry, J. Biol. Chem, doi:10.1016/j.jbc.2021.100847
Fais, Juskeviciene, Francardo, Mateos, Guyard et al., Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia, Int. J. Mol. Sci, doi:10.3390/ijms23074062
Figueroa, Lombardo, Dogliotti, Flynn, Giugliano et al., Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease, Int. J. Gen. Med, doi:10.2147/IJGM.S328486
Fokkens, Scheeren, Upper airway defence mechanisms, Paediatr. Respir. Rev, doi:10.1053/prrv.2000.0073
Greenhalgh, Jimenez, Prather, Tufekci, Fisman et al., Ten scientific reasons in support of airborne transmission of SARS-CoV-2, Lancet, doi:10.1016/S0140-6736(21)00869-2
Guleria, Krishan, Sharma, Kanchan, Impact of prolonged wearing of face masks-Medical and forensic implications, J. Infect. Dev. Ctries, doi:10.3855/jidc.16618
Havervall, Marking, Svensson, Greilert-Norin, Bacchus et al., Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection, N. Engl. J. Med, doi:10.1056/NEJMc2209651
Hennings, Thörn, Albinsson, Lingblom, Andersson et al., The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19, Eur. J. Immunol, doi:10.1002/eji.202149655
Hui, Ng, Ho, Yeung, Ching et al., Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract, EBioMedicine, doi:10.1016/j.ebiom.2022.104232
Idrees, Mcgowan, Fawzy, Abuderman, Balasubramaniam et al., Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph191912148
Isho, Abe, Zuo, Jamal, Rathod et al., Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients, Sci. Immunol, doi:10.1126/sciimmunol.abe5511
Iwata-Yoshikawa, Kakizaki, Shiwa-Sudo, Okura, Tahara et al., Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways, Nat. Commun, doi:10.1038/s41467-022-33911-8
Jaimes, Millet, Whittaker, Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site, iScience, doi:10.1016/j.isci.2020.101212
Jessop, Gibson, Lim, Jovic, Combellack et al., A study protocol for a double-blind randomised placebo-controlled trial evaluating the efficacy of carrageenan nasal and throat spray for COVID-19 prophylaxis-ICE-COVID, Trials, doi:10.1186/s13063-022-06685-z
Kozlov, Could a nose spray a day keep COVID away? Nature, doi:10.1038/d41586-022-03341-z
Kozlov, Omicron's feeble attack on the lungs could make it less dangerous, Nature, doi:10.1038/d41586-022-00007-8
Kramer, Eggers, Exner, Hübner, Simon et al., Recommendation of the German Society of Hospital Hygiene (DGKH): Prevention of COVID-19 by virucidal gargling and virucidal nasal spray-Updated version April 2022, GMS Hyg. Infect. Control, doi:10.3205/dgkh000416
Li, Liang, Gao, Ayaz Ahmed, Uy et al., Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis, Am. J. Infect. Control, doi:10.1016/j.ajic.2020.12.007
Li, Yuan, Li, Wang, Spike protein mediated membrane fusion during SARS-CoV-2 infection, J. Med. Virol, doi:10.1002/jmv.28212
Lin, Yue, Yang, Yang, Pan et al., Nasal Spray of Neutralizing Monoclonal Antibody 35B5 Confers Potential Prophylaxis Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern (VOCs): A Small-scale Clinical Trial, Clin. Infect. Dis, doi:10.1093/cid/ciac448
Lippi, Henry, Favaloro, The Benefits of Heparin Use in COVID-19: Pleiotropic Antiviral Activity beyond Anticoagulant and Anti-Inflammatory Properties, Semin. Thromb. Hemost, doi:10.1055/s-0042-1742740
Lippi, Mattiuzzi, Clinical value of anti-SARS-COV-2 serum IgA titration in patients with COVID-19, J. Med. Virol, doi:10.1002/jmv.26539
Lippi, Nocini, Henry, Analysis of online search trends suggests that SARS-CoV-2 Omicron (B.1.1.529) variant causes different symptoms, J. Infect, doi:10.1016/j.jinf.2022.02.011
Lippi, Nocini, Henry, Plebani, Virucidal effects of mouthwashes or mouth rinses: A world of caution for molecular detection of SARS-CoV-2 in saliva, Diagnosis, doi:10.1515/dx-2022-0004
Lippi, Plebani, The novel coronavirus (2019-nCoV) outbreak: Think the unthinkable and be prepared to face the challenge, Diagnosis, doi:10.1515/dx-2020-0015
Lippi, Sanchis-Gomar, Henry, Coronavirus disease 2019 (COVID-19): The portrait of a perfect storm, Ann. Transl. Med, doi:10.21037/atm.2020.03.157
Lorenzo-Redondo, Ozer, Hultquist, Covid-19: Is omicron less lethal than delta?, BMJ, doi:10.1136/bmj.o1806
Lu, Yin, Pei, Zhang, Qu et al., Nasal delivery of broadly neutralizing antibodies protects mice from lethal challenge with SARS-CoV-2 delta and omicron variants, Virol. Sin, doi:10.1016/j.virs.2022.02.005
Malato, Ribeiro, Leite, Casaca, Fernandes et al., Risk of BA.5 Infection among Persons Exposed to Previous SARS-CoV-2 Variants, N. Engl. J. Med, doi:10.1056/NEJMc2209479
Mcmahan, Giffin, Tostanoski, Chung, Siamatu et al., Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters, Med, doi:10.1016/j.medj.2022.03.004
Meggiolaro, Sane Schepisi, Farina, Castagna, Mammone et al., Effectiveness of vaccination against SARS-CoV-2 Omicron variant infection, symptomatic disease, and hospitalization: A systematic review and meta-analysis, Expert Rev. Vaccines, doi:10.1080/14760584.2022.2130773
Moakes, Davies, Stamataki, Grover, Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2, Adv. Mater, doi:10.1002/adma.202008304
Morokutti-Kurz, Fröba, Graf, Große, Grassauer et al., Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro, PLoS ONE, doi:10.1371/journal.pone.0237480
Nasrallah, Do preexisting antibodies against seasonal coronaviruses have a protective role against SARS-CoV-2 infections and impact on COVID-19 severity?, EBioMedicine, doi:10.1016/j.ebiom.2022.103831
Ning, Huang, Youngquist, Scott, Niu et al., Liposome-mediated detection of SARS-CoV-2 RNA-positive extracellular vesicles in plasma, Nat. Nanotechnol, doi:10.1038/s41565-021-00939-8
Nocini, Henry, Mattiuzzi, Lippi, Evolution of throat symptoms during the COVID-19 pandemic in the US, Diagnosis, doi:10.1515/dx-2022-0084
Paolacci, Ergoren, De Forni, Manara, Poddesu et al., In vitro and clinical studies on the efficacy of α-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202112_27337
Paull, Luscombe, Castellarnau, Heery, Bobardt et al., Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice, Viruses, doi:10.3390/v13081656
Planas, Staropoli, Porot, Guivel-Benhassine, Handala et al., Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection, Med, doi:10.1016/j.medj.2022.09.010
Posch, Vosper, Zaderer, Noureen, Constant et al., ColdZyme Maintains Integrity in SARS-CoV-2-Infected Airway Epithelia, mBio, doi:10.1128/mBio.00904-21
Pyrć, Milewska, Duran, Botwina, Dabrowska et al., SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an ant-Viral nasal spray, Sci. Rep, doi:10.1038/s41598-021-99404-8
Ragull, Núñez-Gómez, Aretxalde, Zabala, Párraga-Niño et al., Low risk of environmental contagion by SARS-CoV-2 in non-sanitary spaces, Enferm. Infecc. Microbiol. Clin, doi:10.1016/j.eimc.2022.01.015
Sampath, Khedr, Qamar, Tekin, Singh et al., Pandemics Throughout the History, Cureus, doi:10.7759/cureus.18136
Sano, Bhavsar, Singh, Floda, Srivastava et al., SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals, Nat. Commun, doi:10.1038/s41467-022-32389-8
Semeraro, Gaetano, Zupin, Poloni, Merlach et al., Operative Protocol for Testing the Efficacy of Nasal Filters in Preventing Airborne Transmission of SARS-CoV-2, Int. J. Environ. Res. Public Health
Shapira, Monreal, Dion, Buchholz, Imbiakha et al., A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic, Nature, doi:10.1038/s41586-022-04661-w
Shragai, Pratt, Castro Georgi, Donnelly, Schwartz et al., Household characteristics associated with surface contamination of SARS-CoV-2 and frequency of RT-PCR and viral culture positivity-California and Colorado, PLoS ONE, doi:10.1371/journal.pone.0274946
Tran, Mostafa, Tawfik, Soliman, Mahabir et al., Efficacy of face masks against respiratory infectious diseases: A systematic review and network analysis of randomizedcontrolled trials, J. Breath Res, doi:10.1088/1752-7163/ac1ea5
Wang, Bowen, Valdez, Gherasim, Gordon et al., Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot, bioRxiv, doi:10.1101/2022.10.22.513349
Yip, Lee, Ng, Xu, Yung et al., An anti-inflammatory and anti-fibrotic proprietary Chinese medicine nasal spray designated as Allergic Rhinitis Nose Drops (ARND) with potential to prevent SARS-CoV-2 coronavirus infection by targeting RBD (Delta)-angiotensin converting enzyme 2 (ACE2) binding, Chin. Med, doi:10.1186/s13020-022-00635-2
Zaderer, Dichtl, Bellmann-Weiler, Lass-Flörl, Posch et al., ColdZyme ® protects airway epithelia from infection with BA.4/5, Respir. Res, doi:10.1186/s12931-022-02223-2
Zeng, Evans, King, Zheng, Oltz et al., SARS-CoV-2 spreads through cell-to-cell transmission, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2111400119
DOI record:
{
"DOI": "10.3390/biomedicines10112966",
"ISSN": [
"2227-9059"
],
"URL": "http://dx.doi.org/10.3390/biomedicines10112966",
"abstract": "<jats:p>Airborne pathogens, including SARS-CoV-2, are mainly contracted within the airway pathways, especially in the nasal epithelia, where inhaled air is mostly filtered in resting conditions. Mucosal immunity developing after SARS-CoV-2 infection or vaccination in this part of the body represents one of the most efficient deterrents for preventing viral infection. Nonetheless, the complete lack of such protection in SARS-CoV-2 naïve or seronegative subjects, the limited capacity of neutralizing new and highly mutated lineages, along with the progressive waning of mucosal immunity over time, lead the way to considering alternative strategies for constructing new walls that could stop or entrap the virus at the nasal mucosa surface, which is the area primarily colonized by the new SARS-CoV-2 Omicron sublineages. Among various infection preventive strategies, those based on generating physical barriers within the nose, aimed at impeding host cell penetration (i.e., using compounds with mucoadhesive properties, which act by hindering, entrapping or adsorbing the virus), or those preventing the association of SARS-CoV-2 with its cellular receptors (i.e., administering anti-SARS-CoV-2 neutralizing antibodies or agents that inhibit priming or binding of the spike protein) could be considered appealing perspectives. Provided that these agents are proven safe, comfortable, and compatible with daily life, we suggest prioritizing their usage in subjects at enhanced risk of contagion, during high-risk activities, as well as in patients more likely to develop severe forms of SARS-CoV-2 infection.</jats:p>",
"alternative-id": [
"biomedicines10112966"
],
"author": [
{
"affiliation": [
{
"name": "Unit of Otorhinolaryngology, Department of Surgery, Dentistry, Paediatrics and Gynaecology, University of Verona, Piazzale L.A. Scuro 10, 37134 Verona, Italy"
}
],
"family": "Nocini",
"given": "Riccardo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Nephrology and Hypertension, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229, USA"
}
],
"family": "Henry",
"given": "Brandon",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5902-2525",
"affiliation": [
{
"name": "Service of Clinical Governance, Provincial Agency for Social and Sanitary Services (APSS), Via Alcide Degasperi 79, 38123 Trento, Italy"
}
],
"authenticated-orcid": false,
"family": "Mattiuzzi",
"given": "Camilla",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9523-9054",
"affiliation": [
{
"name": "Section of Clinical Biochemistry and School of Medicine, University of Verona, Piazzale L.A. Scuro 10, 37134 Verona, Italy"
}
],
"authenticated-orcid": false,
"family": "Lippi",
"given": "Giuseppe",
"sequence": "additional"
}
],
"container-title": "Biomedicines",
"container-title-short": "Biomedicines",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
18
]
],
"date-time": "2022-11-18T03:57:44Z",
"timestamp": 1668743864000
},
"deposited": {
"date-parts": [
[
2025,
7,
11
]
],
"date-time": "2025-07-11T10:59:40Z",
"timestamp": 1752231580000
},
"indexed": {
"date-parts": [
[
2025,
7,
12
]
],
"date-time": "2025-07-12T01:22:54Z",
"timestamp": 1752283374116,
"version": "3.41.2"
},
"is-referenced-by-count": 10,
"issue": "11",
"issued": {
"date-parts": [
[
2022,
11,
17
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T00:00:00Z",
"timestamp": 1668643200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2227-9059/10/11/2966/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2966",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
11,
17
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
17
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1515/dx-2020-0015",
"article-title": "The novel coronavirus (2019-nCoV) outbreak: Think the unthinkable and be prepared to face the challenge",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Diagnosis",
"key": "ref_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020). The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol., 5, 536–544."
},
{
"article-title": "WHO Declares COVID-19 a Pandemic",
"author": "Cucinotta",
"first-page": "157",
"journal-title": "Acta Biomed.",
"key": "ref_3",
"volume": "91",
"year": "2020"
},
{
"article-title": "Pandemics Throughout the History",
"author": "Sampath",
"first-page": "e18136",
"journal-title": "Cureus",
"key": "ref_4",
"volume": "13",
"year": "2021"
},
{
"key": "ref_5",
"unstructured": "World Health Organization (2022, November 06). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/."
},
{
"DOI": "10.1016/S0140-6736(21)00869-2",
"article-title": "Ten scientific reasons in support of airborne transmission of SARS-CoV-2",
"author": "Greenhalgh",
"doi-asserted-by": "crossref",
"first-page": "1603",
"journal-title": "Lancet",
"key": "ref_6",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.eimce.2022.09.004",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Ragull, S., Núñez-Gómez, A., Aretxalde, M.C., Zabala, N., Párraga-Niño, N., and Sabrià, M. (Enferm. Infecc. Microbiol. Clin., 2022). Low risk of environmental contagion by SARS-CoV-2 in non-sanitary spaces, Enferm. Infecc. Microbiol. Clin., ahead of print."
},
{
"DOI": "10.1371/journal.pone.0274946",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Shragai, T., Pratt, C., Castro Georgi, J., Donnelly, M.A.P., Schwartz, N.G., Soto, R., Chuey, M., Chu, V.T., Marcenac, P., and Park, G.W. (2022). Household characteristics associated with surface contamination of SARS-CoV-2 and frequency of RT-PCR and viral culture positivity-California and Colorado, 2021. PLoS ONE, 17."
},
{
"DOI": "10.21037/atm.2020.03.157",
"article-title": "Coronavirus disease 2019 (COVID-19): The portrait of a perfect storm",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Ann. Transl. Med.",
"key": "ref_9",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2020.101212",
"article-title": "Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site",
"author": "Jaimes",
"doi-asserted-by": "crossref",
"first-page": "101212",
"journal-title": "iScience",
"key": "ref_10",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1016/j.jbc.2021.100847",
"article-title": "Role of host factors in SARS-CoV-2 entry",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "100847",
"journal-title": "J. Biol. Chem.",
"key": "ref_11",
"volume": "297",
"year": "2021"
},
{
"DOI": "10.1038/d41586-022-00007-8",
"article-title": "Omicron’s feeble attack on the lungs could make it less dangerous",
"author": "Kozlov",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Nature",
"key": "ref_12",
"volume": "601",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2022.104232",
"article-title": "Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract",
"author": "Hui",
"doi-asserted-by": "crossref",
"first-page": "104232",
"journal-title": "EBioMedicine",
"key": "ref_13",
"volume": "83",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-33911-8",
"article-title": "Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways",
"author": "Kakizaki",
"doi-asserted-by": "crossref",
"first-page": "6100",
"journal-title": "Nat. Commun.",
"key": "ref_14",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2111400119",
"article-title": "SARS-CoV-2 spreads through cell-to-cell transmission",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "e2111400119",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_15",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28212",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Li, X., Yuan, H., Li, X., and Wang, H. (J. Med. Virol., 2022). Spike protein mediated membrane fusion during SARS-CoV-2 infection, J. Med. Virol., ahead of print."
},
{
"DOI": "10.1038/s41565-021-00939-8",
"article-title": "Liposome-mediated detection of SARS-CoV-2 RNA-positive extracellular vesicles in plasma",
"author": "Ning",
"doi-asserted-by": "crossref",
"first-page": "1039",
"journal-title": "Nat. Nanotechnol.",
"key": "ref_17",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1515/dx-2022-0084",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Nocini, R., Henry, B.M., Mattiuzzi, C., and Lippi, G. (Diagnosis, 2022). Evolution of throat symptoms during the COVID-19 pandemic in the US, Diagnosis, ahead of print."
},
{
"DOI": "10.1016/j.jinf.2022.02.011",
"article-title": "Analysis of online search trends suggests that SARS-CoV-2 Omicron (B.1.1.529) variant causes different symptoms",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "e76",
"journal-title": "J. Infect.",
"key": "ref_19",
"volume": "84",
"year": "2022"
},
{
"DOI": "10.1016/0013-9351(80)90042-0",
"article-title": "Nose or mouth breathing?",
"author": "Camner",
"doi-asserted-by": "crossref",
"first-page": "394",
"journal-title": "Environ. Res.",
"key": "ref_20",
"volume": "21",
"year": "1980"
},
{
"DOI": "10.1002/jmv.26539",
"article-title": "Clinical value of anti-SARS-COV-2 serum IgA titration in patients with COVID-19",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "1210",
"journal-title": "J. Med. Virol.",
"key": "ref_21",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-18058-8",
"article-title": "A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction",
"author": "Ejemel",
"doi-asserted-by": "crossref",
"first-page": "4198",
"journal-title": "Nat. Commun.",
"key": "ref_22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/eji.202149655",
"article-title": "The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19",
"author": "Hennings",
"doi-asserted-by": "crossref",
"first-page": "800",
"journal-title": "Eur. J. Immunol.",
"key": "ref_23",
"volume": "52",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2209651",
"article-title": "Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection",
"author": "Havervall",
"doi-asserted-by": "crossref",
"first-page": "1333",
"journal-title": "N. Engl. J. Med.",
"key": "ref_24",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2022.103831",
"article-title": "Do preexisting antibodies against seasonal coronaviruses have a protective role against SARS-CoV-2 infections and impact on COVID-19 severity?",
"author": "Nasrallah",
"doi-asserted-by": "crossref",
"first-page": "103831",
"journal-title": "EBioMedicine",
"key": "ref_25",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2209479",
"article-title": "Risk of BA.5 Infection among Persons Exposed to Previous SARS-CoV-2 Variants",
"author": "Malato",
"doi-asserted-by": "crossref",
"first-page": "953",
"journal-title": "N. Engl. J. Med.",
"key": "ref_26",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2209306",
"article-title": "Protective Effect of Previous SARS-CoV-2 Infection against Omicron BA.4 and BA.5 Subvariants",
"author": "Altarawneh",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "N. Engl. J. Med.",
"key": "ref_27",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.2139/ssrn.4147520",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Meggiolaro, A., Sane Schepisi, M., Farina, S., Castagna, C., Mammone, A., Siddu, A., Stefanelli, P., Boccia, S., and Rezza, G. (Expert Rev. Vaccines, 2022). Effectiveness of vaccination against SARS-CoV-2 Omicron variant infection, symptomatic disease, and hospitalization: A systematic review and meta-analysis, Expert Rev. Vaccines, ahead of print."
},
{
"DOI": "10.1101/2022.10.22.513349",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Wang, Q., Bowen, A., Valdez, R., Gherasim, C., Gordon, A., Liu, L., and Ho, D.D. (2022). Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot. bioRxiv."
},
{
"DOI": "10.1126/sciimmunol.abe5511",
"article-title": "Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients",
"author": "Isho",
"doi-asserted-by": "crossref",
"first-page": "eabe5511",
"journal-title": "Sci. Immunol.",
"key": "ref_30",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41467-022-32389-8",
"article-title": "SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals",
"author": "Sano",
"doi-asserted-by": "crossref",
"first-page": "5135",
"journal-title": "Nat. Commun.",
"key": "ref_31",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1101/2022.07.22.22277885",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Planas, D., Staropoli, I., Porot, F., Guivel-Benhassine, F., Handala, L., Prot, M., Bolland, W.H., Puech, J., Péré, H., and Veyer, D. (Med, 2022). Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection, Med, ahead of print."
},
{
"DOI": "10.1515/dx-2022-0004",
"article-title": "Virucidal effects of mouthwashes or mouth rinses: A world of caution for molecular detection of SARS-CoV-2 in saliva",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "Diagnosis",
"key": "ref_33",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3390/ijerph191912148",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Idrees, M., McGowan, B., Fawzy, A., Abuderman, A.A., Balasubramaniam, R., and Kujan, O. (2022). Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.1016/j.ajic.2020.12.007",
"article-title": "Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "900",
"journal-title": "Am. J. Infect. Control.",
"key": "ref_35",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1088/1752-7163/ac1ea5",
"article-title": "Efficacy of face masks against respiratory infectious diseases: A systematic review and network analysis of randomized-controlled trials",
"author": "Tran",
"doi-asserted-by": "crossref",
"first-page": "047102",
"journal-title": "J. Breath Res.",
"key": "ref_36",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.3855/jidc.16618",
"article-title": "Impact of prolonged wearing of face masks—Medical and forensic implications",
"author": "Guleria",
"doi-asserted-by": "crossref",
"first-page": "1578",
"journal-title": "J. Infect. Dev. Ctries.",
"key": "ref_37",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1038/d41586-022-03341-z",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Kozlov, M. (Nature, 2022). Could a nose spray a day keep COVID away?, Nature, ahead of print."
},
{
"DOI": "10.1126/science.abf4896",
"article-title": "Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets",
"author": "Schmitz",
"doi-asserted-by": "crossref",
"first-page": "1379",
"journal-title": "Science",
"key": "ref_39",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04661-w",
"article-title": "A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic",
"author": "Shapira",
"doi-asserted-by": "crossref",
"first-page": "340",
"journal-title": "Nature",
"key": "ref_40",
"volume": "605",
"year": "2022"
},
{
"DOI": "10.1016/j.virs.2022.02.005",
"article-title": "Nasal delivery of broadly neutralizing antibodies protects mice from lethal challenge with SARS-CoV-2 delta and omicron variants",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "Virol. Sin.",
"key": "ref_41",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1186/s13020-022-00635-2",
"article-title": "An anti-inflammatory and anti-fibrotic proprietary Chinese medicine nasal spray designated as Allergic Rhinitis Nose Drops (ARND) with potential to prevent SARS-CoV-2 coronavirus infection by targeting RBD (Delta)- angiotensin converting enzyme 2 (ACE2) binding",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "88",
"journal-title": "Chin. Med.",
"key": "ref_42",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3390/ijms23074062",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Fais, F., Juskeviciene, R., Francardo, V., Mateos, S., Guyard, M., Viollet, C., Constant, S., Borelli, M., and Hohenfeld, I.P. (2022). Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3390/v13081656",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Paull, J.R.A., Luscombe, C.A., Castellarnau, A., Heery, G.P., Bobardt, M.D., and Gallay, P.A. (2021). Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses, 13."
},
{
"DOI": "10.3390/v13122345",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Bentley, K., and Stanton, R.J. (2021). Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses, 13."
},
{
"article-title": "Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia",
"author": "Bovard",
"first-page": "101187",
"journal-title": "Biochem. Biophys. Rep.",
"key": "ref_46",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0237480",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Morokutti-Kurz, M., Fröba, M., Graf, P., Große, M., Grassauer, A., Auth, J., Schubert, U., and Prieschl-Grassauer, E. (2021). Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE, 16."
},
{
"DOI": "10.1002/adma.202008304",
"article-title": "Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2",
"author": "Moakes",
"doi-asserted-by": "crossref",
"first-page": "e2008304",
"journal-title": "Adv. Mater.",
"key": "ref_48",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-99404-8",
"article-title": "SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an ant—Viral nasal spray",
"author": "Milewska",
"doi-asserted-by": "crossref",
"first-page": "20012",
"journal-title": "Sci. Rep.",
"key": "ref_49",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12931-022-02223-2",
"article-title": "ColdZyme® protects airway epithelia from infection with BA.4/5",
"author": "Zaderer",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Respir. Res.",
"key": "ref_50",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1128/mBio.00904-21",
"article-title": "ColdZyme Maintains Integrity in SARS-CoV-2-Infected Airway Epithelia",
"author": "Posch",
"doi-asserted-by": "crossref",
"first-page": "e00904-21",
"journal-title": "mBio",
"key": "ref_51",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S328486",
"article-title": "Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease",
"author": "Figueroa",
"doi-asserted-by": "crossref",
"first-page": "6277",
"journal-title": "Int. J. Gen. Med.",
"key": "ref_52",
"volume": "14",
"year": "2021"
},
{
"article-title": "In vitro and clinical studies on the efficacy of α-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection",
"author": "Paolacci",
"first-page": "81",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_53",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac448",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Lin, Y., Yue, S., Yang, Y., Yang, S., Pan, Z., Yang, X., Gao, L., Zhou, J., Li, Z., and Hu, L. (Clin. Infect. Dis., 2022). Nasal Spray of Neutralizing Monoclonal Antibody 35B5 Confers Potential Prophylaxis Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern (VOCs): A Small-scale Clinical Trial, Clin. Infect. Dis., ahead of print."
},
{
"DOI": "10.1055/s-0042-1742740",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Lippi, G., Henry, B.M., and Favaloro, E.J. (Semin. Thromb. Hemost., 2022). The Benefits of Heparin Use in COVID-19: Pleiotropic Antiviral Activity beyond Anticoagulant and Anti-Inflammatory Properties, Semin. Thromb. Hemost., ahead of print."
},
{
"DOI": "10.1055/s-0042-1749395",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Carpenè, G., Negrini, D., Lippi, G., Favaloro, E.J., and Montagnana, M. (Semin. Thromb. Hemost., 2022). Heparin: The Journey from Parenteral Agent to Nasal Delivery, Semin. Thromb. Hemost., ahead of print."
},
{
"DOI": "10.1128/mbio.02558-22",
"article-title": "Inhalation of Low Molecular Weight Heparins as Prophylaxis against SARS-CoV-2",
"author": "Eder",
"doi-asserted-by": "crossref",
"first-page": "e0255822",
"journal-title": "mBio",
"key": "ref_57",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1186/s13063-022-06685-z",
"article-title": "A study protocol for a double-blind randomised placebo-controlled trial evaluating the efficacy of carrageenan nasal and throat spray for COVID-19 prophylaxis-ICE-COVID",
"author": "Jessop",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "Trials",
"key": "ref_58",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.7319",
"article-title": "Risk of Infection and Hospitalization Among Vaccinated and Unvaccinated Children and Adolescents in New York After the Emergence of the Omicron Variant",
"author": "Dorabawila",
"doi-asserted-by": "crossref",
"first-page": "2242",
"journal-title": "JAMA",
"key": "ref_59",
"volume": "327",
"year": "2022"
},
{
"article-title": "Upper airway defence mechanisms",
"author": "Fokkens",
"first-page": "336",
"journal-title": "Paediatr. Respir. Rev.",
"key": "ref_60",
"volume": "1",
"year": "2000"
},
{
"article-title": "Covid-19: Is omicron less lethal than delta?",
"author": "Ozer",
"first-page": "o1806",
"journal-title": "BMJ",
"key": "ref_61",
"volume": "378",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-31200-y",
"article-title": "SARS-CoV-2 Omicron variant causes mild pathology in the upper and lower respiratory tract of hamsters",
"author": "Armando",
"doi-asserted-by": "crossref",
"first-page": "3519",
"journal-title": "Nat. Commun.",
"key": "ref_62",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.medj.2022.03.004",
"article-title": "Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters",
"author": "McMahan",
"doi-asserted-by": "crossref",
"first-page": "262",
"journal-title": "Med",
"key": "ref_63",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/d41591-022-00106-z",
"doi-asserted-by": "crossref",
"key": "ref_64",
"unstructured": "Carvalho, T. (Nat. Med., 2022). Intranasal COVID-19 vaccine fails to induce mucosal immunity, Nat. Med., ahead of print."
},
{
"article-title": "Recommendation of the German Society of Hospital Hygiene (DGKH): Prevention of COVID-19 by virucidal gargling and virucidal nasal spray—Updated version April 2022",
"author": "Kramer",
"first-page": "Doc13",
"journal-title": "GMS Hyg. Infect. Control.",
"key": "ref_65",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3390/ijerph192113790",
"doi-asserted-by": "crossref",
"key": "ref_66",
"unstructured": "Semeraro, S., Gaetano, A.S., Zupin, L., Poloni, C., Merlach, E., Greco, E., Licen, S., Fontana, F., Leo, S., and Miani, A. (2022). Operative Protocol for Testing the Efficacy of Nasal Filters in Preventing Airborne Transmission of SARS-CoV-2. Int. J. Environ. Res. Public Health, 19."
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2227-9059/10/11/2966"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Improving Nasal Protection for Preventing SARS-CoV-2 Infection",
"type": "journal-article",
"updated-by": [
{
"DOI": "10.3390/biomedicines13071698",
"label": "Correction",
"source": "publisher",
"type": "correction",
"updated": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T00:00:00Z",
"timestamp": 1668643200000
}
}
],
"volume": "10"
}