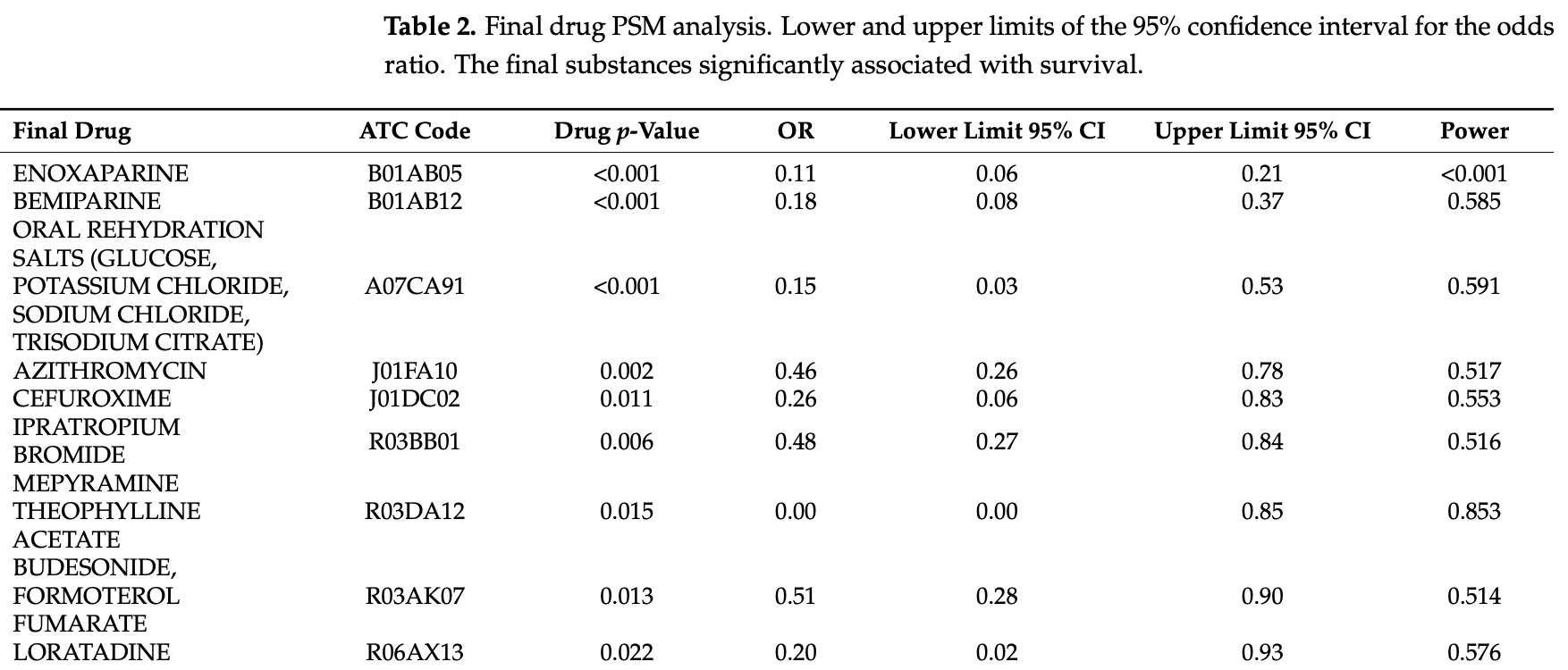
A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis
et al., Pharmaceuticals, doi:10.3390/ph15010078, Jan 2022
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 3,712 hospitalized patients in Spain, showing lower mortality with existing use of loratadine.
|
risk of death, 80.0% lower, OR 0.20, p = 0.05, loratadine, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Monserrat Villatoro et al., 8 Jan 2022, retrospective, propensity score matching, Spain, peer-reviewed, 18 authors.
A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis
Pharmaceuticals, doi:10.3390/ph15010078
Data from several cohorts of coronavirus disease 2019 suggest that the most common comorbidities for severe COVID-19 disease are the elderly, high blood pressure, and diabetes; however, it is not currently known whether the previous use of certain drugs help or hinder recovery. This study aims to explore the association of previous hospitalisation use of medication on the mortality of COVID-19 disease. A retrospective case-control from two hospitals in Madrid, Spain, included all patients aged 18 years or above hospitalised with a diagnosis of COVID-19. A Propensity Score matching (PSM) analysis was performed. Confounding variables were considered to be age, sex, and the number of comorbidities. Finally, 3712 patients were included. Of these, 687 (18.5%) patients died (cases). The 22,446 medicine trademarks used previous to admission were classified according to the ATC, obtaining 689 final drugs; all of them were included in PSM analysis. Eleven drugs displayed a reduction in mortality: azithromycin, bemiparine, budesonide-formoterol fumarate, cefuroxime, colchicine, enoxaparin, ipratropium bromide, loratadine, mepyramine theophylline acetate, oral rehydration salts, and salbutamol sulphate. Eight final drugs displayed an increase in mortality: acetylsalicylic acid, digoxin, folic acid, mirtazapine, linagliptin, enalapril, atorvastatin, and allopurinol. Medication associated with survival (anticoagulants, antihistamines, azithromycin, bronchodilators, cefuroxime, colchicine, and inhaled corticosteroids) may be candidates for future clinical trials. Drugs associated with mortality show an interaction with the underlying conditions.
Conflicts of Interest: The authors declare no conflict of interest.
References
Al-Khafaji, Al-Duhaidahawi, Taskin Tok, Using integrated computational approaches to identify safe and rapid treatment for SARS-CoV-2, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1764392
Atc/Ddd, None
Batiha, Gari, Elshony, Shaheen, Abubakar et al., Hypertension and its management in COVID-19 patients: The assorted view, Int. J. Cardiol. Cardiovasc. Risk Prev, doi:10.1016/j.ijcrp.2021.200121
Bean, Kraljevic, Searle, Bendayan, Pickles et al., Treatment with AVE-inhibitors is associated with less severe disease with SARS-COVID-19 infection in a multi-site UK acute Hospital Trust, MedRxiv, doi:10.1101/2020.04.07.20056788
Borobia, Carcas, Arnalich, Álvarez-Sala, Monserrat-Villatoro et al., A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe, J. Clin. Med, doi:10.3390/jcm9061733
Cho, Lee, Kim, Kim, Kim et al., Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19, Sci. Rep, doi:10.1038/s41598-020-72879-7
Coupland, Dhiman, Morriss, Arthur, Barton et al., Antidepressant use and risk of adverse outcomes in older people: Population based cohort study, BMJ, doi:10.1136/bmj.d4551
De Abajo, Rodríguez-Martín, Lerma, Mejía-Abril, Aguilar et al., MED-ACE2-COVID-19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study, Lancet, doi:10.1016/S0140-6736(20)31030-8
Durojaiye, Clarke, Stamatiades, Wang, Repurposing cefuroxime for treatment of COVID-19: A scoping review of in silico studies, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1777904
Echeverría-Esnal, Martin-Ontiyuelo, Navarrete-Rouco, Cuscó, Ferrández et al., Azithromycin in the treatment of COVID-19: A review, Expert Rev. Anti Infect. Ther, doi:10.1080/14787210.2020.1813024
Elfiky, SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: An in silico perspective, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1761882
Fedele, De Francesco, Riso, Collo, Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview, Nutrition, doi:10.1016/j.nut.2020.111016
Feingold, Anawalt, Boyce, Chrousos, De Herder et al., Lipid and Lipoprotein Levels in Patients with COVID-19 Infections
Foundation, A Language and Environment for Statistical Computing; R Foundation for Statistical Computing
Garg, Kim, Whitaker, O'halloran, Cummings et al., Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019-COVID-NET, Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm6915e3
Goyal, Choi, Pinheiro, Schenck, Chen et al., Clinical Characteristics of COVID-19 in New York City, N. Engl. J. Med, doi:10.1056/NEJMc2010419
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Clin. Exp. Pharmacol. Physiol, doi:10.1111/1440-1681.13488
Hou, Ge, Li, Wang, He et al., Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis, Chem. Interact, doi:10.1016/j.cbi.2021.109420
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jarcho, Ingelfinger, Hamel, ; D'agostino, Sr et al., Inhibitors of the Renin-Angiotensin-Aldosterone System and COVID-19, N. Engl. J. Med, doi:10.1056/NEJMe2012924
Khan, Siddique, Shereen, Ali, Liu et al., Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options, J. Clin. Microbiol, doi:10.1128/JCM.00187-20
Kilis-Pstrusinska, Akutko, Braksator, Dancewicz, Grosman-Dziewiszek et al., Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes, J. Clin. Med, doi:10.3390/jcm10235522
Li, De Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat. Rev. Drug Discov
Lu, Drug treatment options for the 2019-new coronavirus (2019-nCoV), Biosci. Trends, doi:10.5582/bst.2020.01020
Mackey, King, Gurley, Kiefer, Liederbauer et al., Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review, Ann. Intern. Med, doi:10.7326/M20-1515
Madan, Siglin, Khan, Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic, Cancer Med, doi:10.1002/cam4.3534
Mdtext, Com, None
Nicolau, Bafadhel, Inhaled corticosteroids in virus pandemics: A treatment for COVID-19?, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30314-3
Ouyang, Lv, Zhong, Wen, Wei et al., Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation, Am. J. Cardiol, doi:10.1016/j.amjcard.2015.01.013
Paar, Wernly, Zhou, Motloch, Hoppe et al., Anti-coagulation for COVID-19 treatment: Both anti-thrombotic and anti-inflammatory?, J. Thromb. Thrombolysis, doi:10.1007/s11239-020-02212-6
Peltzer, Manocha, Ying, Kirzner, Ip et al., Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19, J. Cardiovasc. Electrophysiol, doi:10.1111/jce.14770
Pollard, Blanco, Pollard, Classical Drug Digitoxin Inhibits Influenza Cytokine Storm, with Implications for COVID-19 Therapy, Vivo, doi:10.21873/invivo.12221
Ramakrishnan, Nicolau, Jr, Langford, Mahdi et al., Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00160-0
Reiffel, Propensity Score Matching: The 'Devil is in the Details' Where More May Be Hidden than You Know, Am. J. Med, doi:10.1016/j.amjmed.2019.08.055
Reynolds, Adhikari, Pulgarin, Troxel, Iturrate et al., Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2008975
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area, JAMA
Rishita, Thommana, Ruiz, Ayna, Therapeutic Options for COVID-19: A Review, Cureus, doi:10.7759/cureus.10480
Salah, Mehta, Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19, Am. J. Cardiol
Sammalkorpi, Valtonen, Kerttula, Nikkilä, Taskinen, Changes in serum lipoprotein pattern induced by acute infections, Metabolism, doi:10.1016/0026-0495(88)90120-5
Seon, Kim, Hong, Lim, Oh, Risk of COVID-19 diagnosis and death in patients with mental illness: A cohort study, Epidemiol. Psychiatr. Sci, doi:10.1017/S2045796021000597
Singh, Edwards, Gout management and outcomes during the COVID-19 pandemic: A cross-sectional internet survey, Ther. Adv. Musculoskelet Dis, doi:10.1177/1759720X20966124
Sriram, Insel, A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance, Br. J. Pharmacol, doi:10.1111/bph.15082
Team, RStudio: Integrated Development Environment for R. RStudio
Thompson, Nguyen, Noble, Aronoff, COVID-19-related disease severity in pregnancy, Am. J. Reprod. Immunol, doi:10.1111/aji.13339
Vamos, Erath, Benz, Lopes, Hohnloser, Meta-Analysis of Effects of Digoxin on Survival in Patients with Atrial Fibrillation or Heart Failure: An Update, Am. J. Cardiol, doi:10.1016/j.amjcard.2018.09.036
Vamos, Erath, Hohnloser, Digoxin-associated mortality: A systematic review and meta-analysis of the literature, Eur. Heart J, doi:10.1093/eurheartj/ehv143
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wang, Zhang, Chen, Wang, Zhang et al., Digoxin Is Associated with Increased All-Cause Mortality in Patients with Atrial Fibrillation Regardless of Concomitant Heart Failure: A Meta-Analysis, J. Cardiovasc. Pharmacol, doi:10.1097/FJC.0000000000000274
Zhang, Penninger, Li, Zhong, Slutsky, Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target, Intensive Care Med, doi:10.1007/s00134-020-05985-9
Zhang, Zhu, Cai, Lei, Qin et al., Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized with COVID-19, Circ. Res, doi:10.1161/CIRCRESAHA.120.317134
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3390/ph15010078",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph15010078",
"abstract": "<jats:p>Data from several cohorts of coronavirus disease 2019 (COVID-19) suggest that the most common comorbidities for severe COVID-19 disease are the elderly, high blood pressure, and diabetes; however, it is not currently known whether the previous use of certain drugs help or hinder recovery. This study aims to explore the association of previous hospitalisation use of medication on the mortality of COVID-19 disease. A retrospective case-control from two hospitals in Madrid, Spain, included all patients aged 18 years or above hospitalised with a diagnosis of COVID-19. A Propensity Score matching (PSM) analysis was performed. Confounding variables were considered to be age, sex, and the number of comorbidities. Finally, 3712 patients were included. Of these, 687 (18.5%) patients died (cases). The 22,446 medicine trademarks used previous to admission were classified according to the ATC, obtaining 689 final drugs; all of them were included in PSM analysis. Eleven drugs displayed a reduction in mortality: azithromycin, bemiparine, budesonide-formoterol fumarate, cefuroxime, colchicine, enoxaparin, ipratropium bromide, loratadine, mepyramine theophylline acetate, oral rehydration salts, and salbutamol sulphate. Eight final drugs displayed an increase in mortality: acetylsalicylic acid, digoxin, folic acid, mirtazapine, linagliptin, enalapril, atorvastatin, and allopurinol. Medication associated with survival (anticoagulants, antihistamines, azithromycin, bronchodilators, cefuroxime, colchicine, and inhaled corticosteroids) may be candidates for future clinical trials. Drugs associated with mortality show an interaction with the underlying conditions.</jats:p>",
"alternative-id": [
"ph15010078"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9100-3324",
"affiliation": [],
"authenticated-orcid": false,
"family": "Monserrat Villatoro",
"given": "Jaime",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mejía-Abril",
"given": "Gina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz García",
"given": "Lucía",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6150-4320",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zubiaur",
"given": "Pablo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4925-731X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jiménez González",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandez Jimenez",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cancio",
"given": "Inés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arribas",
"given": "José Ramón",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suarez Fernández",
"given": "Carmen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6173-5711",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mingorance",
"given": "Jesús",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3771-8740",
"affiliation": [],
"authenticated-orcid": false,
"family": "García Rodríguez",
"given": "Julio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villagrasa Ferrer",
"given": "José Ramón",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1823-4174",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carcas",
"given": "Antonio J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frías",
"given": "Jesús",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6519-8885",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abad-Santos",
"given": "Francisco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8584-3263",
"affiliation": [],
"authenticated-orcid": false,
"family": "Borobia",
"given": "Alberto M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4052-8876",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ramírez",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of the COVID@HULP Working Group and Other Collaborators from Hospital Universitario de la Princesa",
"sequence": "additional"
}
],
"container-title": [
"Pharmaceuticals"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
10
]
],
"date-time": "2022-01-10T04:06:15Z",
"timestamp": 1641787575000
},
"deposited": {
"date-parts": [
[
2022,
1,
20
]
],
"date-time": "2022-01-20T02:34:16Z",
"timestamp": 1642646056000
},
"indexed": {
"date-parts": [
[
2022,
1,
20
]
],
"date-time": "2022-01-20T07:17:16Z",
"timestamp": 1642663036162
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1424-8247"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
8
]
],
"date-time": "2022-01-08T00:00:00Z",
"timestamp": 1641600000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/15/1/78/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "78",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
1,
8
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
8
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMc2010419",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.15585/mmwr.mm6915e3",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1007/s00134-020-05985-9",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1056/NEJMoa2008975",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1056/NEJMe2012924",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317134",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1101/2020.04.07.20056788",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1038/d41573-020-00016-0",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.5582/bst.2020.01020",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1128/JCM.00187-20",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.7759/cureus.10480",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.amjmed.2019.08.055",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1111/jce.14770",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.21873/invivo.12221",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1038/s41598-020-72879-7",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/eurheartj/ehv143",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1097/FJC.0000000000000274",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.amjcard.2015.01.013",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.amjcard.2018.09.036",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.nut.2020.111016",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1111/aji.13339",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"key": "ref27",
"unstructured": "Technical Data Sheet Mirtazapina Cinfa®https://cima.aemps.es/cima/dochtml/ft/67068/FT_67068.html"
},
{
"key": "ref28",
"unstructured": "Technical Data Sheet Qudix®https://cima.aemps.es/cima/dochtml/ft/70169/FT_70169.html"
},
{
"DOI": "10.1136/bmj.d4551",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1017/S2045796021000597",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"key": "ref31",
"unstructured": "Technical Data Sheet Trajenta®https://cima.aemps.es/cima/dochtml/ft/11707004/FT_11707004.html"
},
{
"DOI": "10.3390/jcm10235522",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/S0140-6736(20)31030-8",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.7326/M20-1515",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1111/bph.15082",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.ijcrp.2021.200121",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"article-title": "Lipid and Lipoprotein Levels in Patients with COVID-19 Infections",
"author": "Feingold",
"key": "ref37",
"series-title": "Endotext [Internet]",
"year": "2020"
},
{
"DOI": "10.1016/0026-0495(88)90120-5",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"key": "ref39",
"unstructured": "Technical Data Sheet Veklury®https://cima.aemps.es/cima/dochtml/ft/1201459002/FT_1201459002.html"
},
{
"key": "ref40",
"unstructured": "Technical Data Sheet Kaletra®https://cima.aemps.es/cima/dochtml/ft/01172006/FT_01172006.html"
},
{
"key": "ref41",
"unstructured": "Technical Data Sheet Roactemra®https://cima.aemps.es/cima/dochtml/ft/108492007/FT_108492007.html#4-datos-cl-nicos"
},
{
"DOI": "10.1177/1759720X20966124",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1002/cam4.3534",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1016/j.amjcard.2020.12.073",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1007/s11239-020-02212-6",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1080/14787210.2020.1813024",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1080/07391102.2020.1777904",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1080/07391102.2020.1761882",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1080/07391102.2020.1764392",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1016/S2213-2600(20)30314-3",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/j.cbi.2021.109420",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1111/1440-1681.13488",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"key": "ref54",
"unstructured": "Royal Decree 1090/2015, of 4 December, Regulating Clinical Trials with Medicinal Products, Ethics Committees for Investigation with Medicinal Products and the Spanish Clinical Studies Registryhttps://www.aemps.gob.es/legislacion/espana/investigacionClinica/docs/Royal-Decree-1090-2015_4-December.pdf"
},
{
"key": "ref55",
"unstructured": "Organic Law 3/2018 of 5 December 2018, on the Protection of Personal Data and Guarantee of Digital Rightshttps://noticias.juridicas.com/base_datos/Laboral/632849-lo-3-2018-de-5-dic-proteccion-de-datos-personales-y-garantia-de-los-derechos.html"
},
{
"key": "ref56",
"unstructured": "nCoV Case Records form Version 1.3https://media.tghn.org/medialibrary/2020/03/ISARIC_COVID-19_CRF_V1.3_24Feb2020.pdf"
},
{
"DOI": "10.3390/jcm9061733",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"key": "ref58",
"unstructured": "ATC/DDD Index 2020 Updated 16 December 2019https://www.whocc.no/atc_ddd_index/>"
},
{
"key": "ref59",
"unstructured": "Drugbank Pharmaceutical Knowledge Base that is Enabling Major Advances across the Data-Driven Medicine Industryhttps://www.drugbank.ca/drugs"
},
{
"key": "ref60",
"unstructured": "https://www.R-project.org/"
},
{
"key": "ref61",
"unstructured": "RStudio: Integrated Development Environment for Rhttp://www.rstudio.com/"
}
],
"reference-count": 61,
"references-count": 61,
"relation": {},
"score": 1,
"short-container-title": [
"Pharmaceuticals"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Drug Discovery",
"Pharmaceutical Science",
"Molecular Medicine"
],
"subtitle": [],
"title": [
"A Case-Control of Patients with COVID-19 to Explore the Association of Previous Hospitalisation Use of Medication on the Mortality of COVID-19 Disease: A Propensity Score Matching Analysis"
],
"type": "journal-article",
"volume": "15"
}
monserratvillatoro
