A Phase 3, Randomized, Controlled Trial Evaluating the Efficacy and Safety of Ropeginterferon Alfa-2b in Patients with Moderate COVID-19
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-024-00992-5, NCT05770466, May 2024
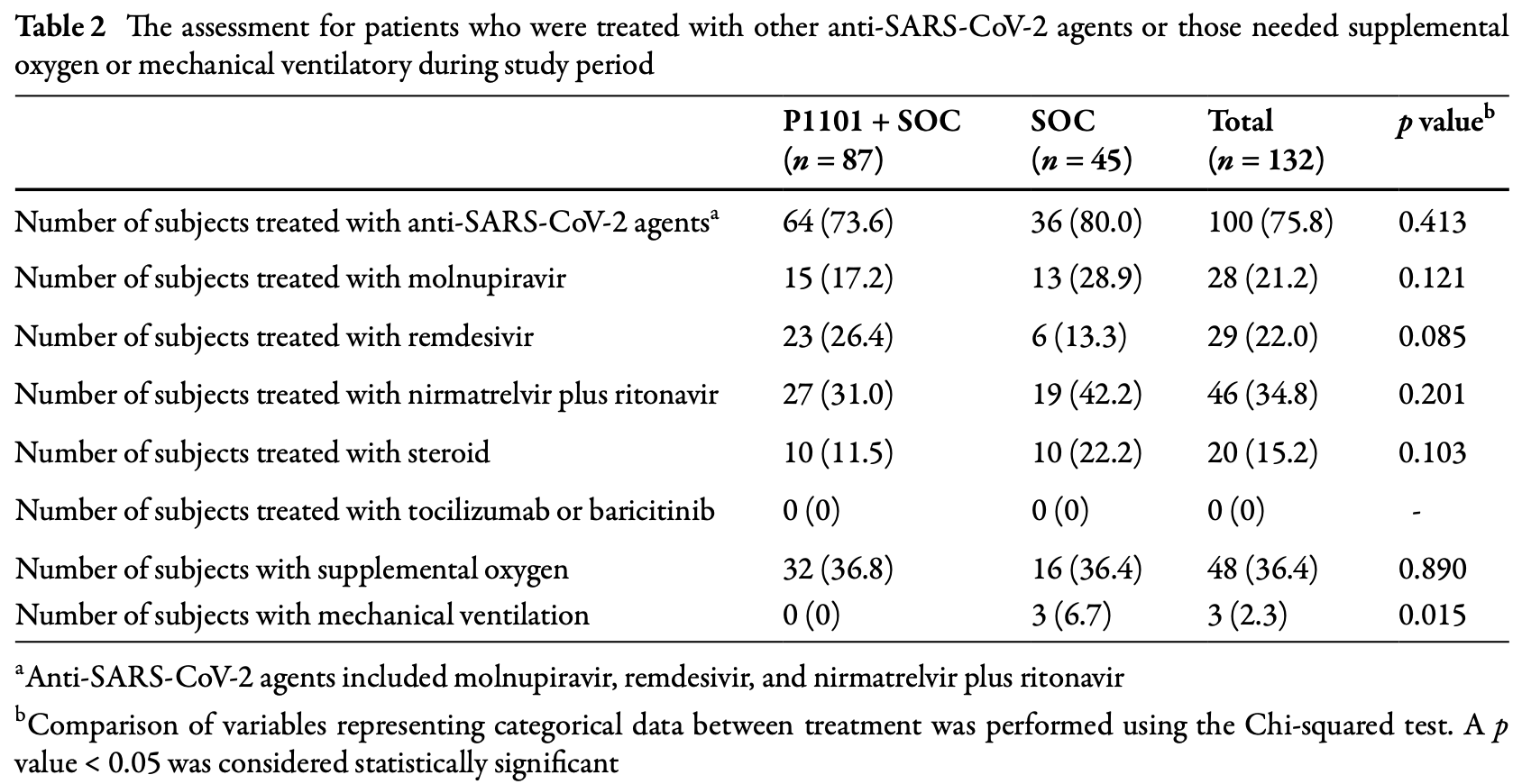
RCT 132 hospitalized moderate COVID-19 patients in Taiwan showing higher rates of viral clearance or discharge by day 11 with ropeginterferon alfa-2b. The ropeginterferon alfa-2b group also showed higher rates of improvement in lung infiltration on day 5 and WHO clinical progression scores on day 8. There was no significant difference in mortality.
|
risk of death, 3.4% higher, RR 1.03, p = 1.00, treatment 2 of 87 (2.3%), control 1 of 45 (2.2%).
|
|
risk of mechanical ventilation, 89.8% lower, RR 0.10, p = 0.04, treatment 0 of 87 (0.0%), control 3 of 45 (6.7%), NNT 15, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 3.4% higher, RR 1.03, p = 1.00, treatment 32 of 87 (36.8%), control 16 of 45 (35.6%).
|
|
risk of no recovery, 3.9% lower, RR 0.96, p = 1.00, treatment 13 of 87 (14.9%), control 7 of 45 (15.6%), NNT 163, Ct<30 and no discharge, day 22.
|
|
risk of no recovery, 48.3% lower, RR 0.52, p = 0.31, treatment 5 of 87 (5.7%), control 5 of 45 (11.1%), NNT 19, Ct<30 and no discharge, day 15.
|
|
risk of no recovery, 59.8% lower, RR 0.40, p = 0.05, treatment 7 of 87 (8.0%), control 9 of 45 (20.0%), NNT 8.4, Ct<30 and no discharge, day 11.
|
|
risk of no recovery, 25.3% lower, RR 0.75, p = 0.47, treatment 13 of 87 (14.9%), control 9 of 45 (20.0%), NNT 20, Ct<30 and no discharge, day 8.
|
|
risk of no recovery, 6.6% lower, RR 0.93, p = 0.70, treatment 56 of 87 (64.4%), control 31 of 45 (68.9%), NNT 22, Ct<30 and no discharge, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Liu et al., 21 May 2024, Randomized Controlled Trial, Taiwan, peer-reviewed, mean age 63.0, 20 authors, study period April 2022 - January 2023, trial NCT05770466 (history).
Contact: whsheng@ntu.edu.tw, 13258@s.tmu.edu.tw.
A Phase 3, Randomized, Controlled Trial Evaluating the Efficacy and Safety of Ropeginterferon Alfa-2b in Patients with Moderate COVID-19
Infectious Diseases and Therapy, doi:10.1007/s40121-024-00992-5
Introduction: Ropeginterferon alfa-2b is a novel mono-pegylated proline-interferon. This clinical study aimed to evaluate its antiviral efficacy of ropeginterferon alfa-2b against SARS-CoV-2 infection. Methods: This is a multicenter, randomized, open-label study. Adult patients with confirmed SARS-CoV-2 infection with initial cycle threshold (Ct) value < 30 and symptom onset within 4 days were enrolled. Eligible patients were randomized in a 2:1 ratio to receive a single 250-µg dose of ropeginterferon alfa-2b subcutaneously plus standard of care (SOC) or to receive SOC alone. The primary endpoint was the proportion of patients with a negative RT-PCR result
Author Contributions. The study was designed by Wang-Huei Sheng, Kang-Yun Lee, Po-Hao Feng, Wang-Da Liu, Albert Qin, and PharmaEssentia clinical team. We would like to thank Jason Liao and Sheena Lin for their help with the statistical design and method. Wang-Da Liu, Po-Hao Feng, Chien-Yu Cheng, Chun-Liang Chou, Chih-Hsin Lee, Min-Chi Lu, Po-Yu Liu, Mei-Hui Lee, Chun-Hsing Liao, Mei-Chuan Chen, Cheng-Pin Chen, Shang-Fu Hsu, Yu-Tien Tzeng, Yi-Chun Lin, Tsong-Yih Ou, Kang-Yun Lee, and Wang-Huei Sheng recruited the patients and collected the data. Chan-Yen Tsai, Albert Qin, Weichung Joe Shih, Po-Hao Feng, Wang-Da Liu, Kang-Yun Lee, and Wang-Huei Sheng analyzed the data and wrote the initial manuscript. All authors interpreted the data and were involved in writing, reviewing, and approving the manuscript. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abd-Elsalam, Salama, Soliman, Naguib, Ibrahim et al., Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial, Am J Trop Med Hyg, doi:10.4269/ajtmh.21-0606
Bhushan, Wanve, Koradia, Bhomia, Soni et al., Efficacy and safety of pegylated interferon-α2b in moderate COVID-19: a phase 3, randomized, comparator-controlled, open-label study, Int J Infect Dis
Bojkova, Rothenburger, Ciesek, Wass, Michaelis et al., SARS-CoV-2 omicron variant virus isolates are highly sensitive to interferon treatment, Cell Discov
Bojkova, Widera, Ciesek, Wass, Michaelis et al., Reduced interferon antagonism but similar drug sensitivity in omicron variant compared to delta variant of SARS-CoV-2 isolates, Cell Res
Chamorro, Tascón, Sanz, Vélez, Nacenta, Radiologic diagnosis of patients with COVID-19, Radiologia
Chen, Chuang, Qin, Zhang, Zhu et al., A phase 3 clinical trial validating the potency and safety of an innovative, extralong-acting interferon in chronic hepatitis C, JGH Open
Chen, Lee, Qin, Luo, Yeh et al., Clinical experience with ropeginterferon alfa-2b in the off-label use for the treatment of COVID-19 patients in Taiwan, Adv Ther
Chow, Shao, Wang, Lokhnygina, Sample Size calculations in clinical research 3rd
Contoli, Papi, Tomassetti, Rizzo, Vieceli et al., Blood interferon-α levels and severity, outcomes, and inflammatory profiles in hospitalized COVID-19 patients, Front Immunol
Feld, Kandel, Biondi, Kozak, Zahoor et al., Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial, Lancet Respir Med
Hadjadj, Yatim, Barnabei, Corneau, Boussier et al., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science
Hoffmann, Hofmann-Winkler, Krüger, Kempf, Nehlmeier et al., SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination, Cell Rep
Hsu, Yu, Su, Peng, Chien et al., Ropeginterferon alfa-2b administered every two weeks for patients with genotype 2 chronic hepatitis C, J Formos Med Assoc
Huang, Hsu, Lu, Yu, Su et al., Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for 1588 Infect Dis Ther (2024) 13:1575-1588 patients with chronic hepatitis B, Hepatol Int
Huang, Qin, Fang, Wang, Tsai et al., Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics, and safety in a phase I clinical trial, Br J Clin Pharmacol
Huang, Qin, Tsai, Chen, Novel pegylated interferon for the treatment of chronic viral hepatitis, Viruses
Huang, Tsai, Tsai, Wang, Zhang et al., Pharmacokinetics and pharmacodynamics of novel long-acting ropeginterferon alpha-2b in healthy Chinese subjects, Adv Ther
Jin, Qin, Zhang, Shen, Wang et al., A phase 2 trial to assess the efficacy and safety of ropeginterferon alfa-2b in Chinese patients with polycythemia vera, Future Oncol
King, Sprent, Dual Nature of Type I Interferons in SARS-CoV-2-Induced Inflammation, Trends Immunol
Lazear, Schoggins, Diamond, Shared and distinct functions of type I and type III interferons, Immunity
Lin, Hsu, Lu, Chuang, Hsu et al., Ropeginterferon alfa-2b in patients with genotype 1 chronic hepatitis C: pharmacokinetics, safety, and preliminary efficacy, JGH Open
Liu, Wang, Shih, Chen, Huang et al., Effect of early dexamethasone on outcomes of COVID-19: A quasi-experimental study using propensity score matching, J Microbiol Immunol Infect
Lokugamage, Hage, Devries, Valero-Jimenez, Schindewolf et al., Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV, J Virol
Mantlo, Bukreyeva, Maruyama, Paessler, Huang, Antiviral activities of type I interferons to SARS-CoV-2 infection, Antiviral Res
Miyachi, Zagrijtschuk, Kang, Yonezu, Qin, Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration, Clin Drug Investig
Moghadasi, Heilmann, Khalil, Nnabuife, Kearns et al., Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors, Sci Adv
Park, Iwasaki, Type I and type III interferons-induction, signaling, evasion, and application to combat COVID-19, Cell Host Microbe
Qin, Urbansky, Yu, Ahmed, Mascrenhas, An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: an overview of previous and ongoing studies with perspectives on the future, Front Oncol
Rahmah, Abarikwu, Arero, Essouma, Jibril et al., Oral antiviral treatments for COVID-19: opportunities and challenges, Pharmacol Rep
Reis, Silva, Silva, Thabane, Campos et al., Early treatment with pegylated interferon lambda for COVID-19, N Engl J Med
Shih, Aisner, Statistical design and analysis of clinical trials: principles and methods
Verstovsek, Komatsu, Gill, Lee, Hou, SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia, Future Oncol
Vitiello, Sars-Cov-2 and risk of antiviral drug resistance, Ir J Med Sci
Vitiello, Zovi, Rezza, New emerging SARS-CoV-2 variants and antiviral agents, Drug Resist Updat
DOI record:
{
"DOI": "10.1007/s40121-024-00992-5",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-024-00992-5",
"alternative-id": [
"992"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "20 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "8 May 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "21 May 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Albert Qin and Chan-Yen Tsai work for PharmaEssentia Corporation. Wang-Huei Sheng serves as a consultant of PharmaEssentia Corporation. The other authors do not have conflict of interest to declare."
},
{
"group": {
"label": "Ethical Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The study was conducted in accordance with the Helsinki Declaration of 2008. The study protocol and informed consent form (ICF) were approved by the Institutional Review Boards of National Taiwan University Hospital, Taipei Medical University, and all other participating hospitals. The committee names and the approval numbers are provided in supplemental Table . ICF including the consents of study participation and publication was obtained from all patients prior to their participation. This study was registered at ClinicalTrials.gov (NCT05770466)."
}
],
"author": [
{
"affiliation": [],
"family": "Liu",
"given": "Wang-Da",
"sequence": "first"
},
{
"affiliation": [],
"family": "Feng",
"given": "Po-Hao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Chien-Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chou",
"given": "Chun-Liang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Chih-Hsin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Min-Chi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Po-Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Mei-Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liao",
"given": "Chun-Hsing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Mei-Chuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Cheng-Pin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hsu",
"given": "Shang-Fu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tzeng",
"given": "Yu-Tien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Yi-Chun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ou",
"given": "Tsong-Yih",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qin",
"given": "Albert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsai",
"given": "Chan-Yen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shih",
"given": "Weichung Joe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Kang-Yun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheng",
"given": "Wang-Huei",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
21
]
],
"date-time": "2024-05-21T12:02:11Z",
"timestamp": 1716292931000
},
"deposited": {
"date-parts": [
[
2024,
7,
2
]
],
"date-time": "2024-07-02T05:28:20Z",
"timestamp": 1719898100000
},
"funder": [
{
"name": "PharmaEssentia Corporation"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
3
]
],
"date-time": "2024-07-03T00:13:25Z",
"timestamp": 1719965605795
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2024,
5,
21
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2024,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
21
]
],
"date-time": "2024-05-21T00:00:00Z",
"timestamp": 1716249600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
21
]
],
"date-time": "2024-05-21T00:00:00Z",
"timestamp": 1716249600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-00992-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-024-00992-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-00992-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "1575-1588",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2024,
5,
21
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
21
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1126/sciadv.ade8778",
"doi-asserted-by": "crossref",
"key": "992_CR1",
"unstructured": "Moghadasi SA, Heilmann E, Khalil AM, Nnabuife C, Kearns FL, Ye C, et al. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. Sci Adv 2023;9:eade8778."
},
{
"DOI": "10.1007/s11845-021-02820-y",
"author": "A Vitiello",
"doi-asserted-by": "publisher",
"first-page": "2367",
"journal-title": "Ir J Med Sci",
"key": "992_CR2",
"unstructured": "Vitiello A. Sars-Cov-2 and risk of antiviral drug resistance. Ir J Med Sci. 2022;191:2367–8.",
"volume": "191",
"year": "2022"
},
{
"DOI": "10.1007/s43440-022-00388-7",
"author": "L Rahmah",
"doi-asserted-by": "publisher",
"first-page": "1255",
"journal-title": "Pharmacol Rep",
"key": "992_CR3",
"unstructured": "Rahmah L, Abarikwu SO, Arero AG, Essouma M, Jibril AT, Fal A, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep. 2022;74:1255–78.",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2021.109415",
"doi-asserted-by": "crossref",
"key": "992_CR4",
"unstructured": "Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep 2021;36:109415."
},
{
"DOI": "10.1016/j.it.2021.02.003",
"author": "C King",
"doi-asserted-by": "publisher",
"first-page": "312",
"journal-title": "Trends Immunol",
"key": "992_CR5",
"unstructured": "King C, Sprent J. Dual Nature of Type I Interferons in SARS-CoV-2-Induced Inflammation. Trends Immunol. 2021;42:312–22.",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.08.044",
"author": "BLS Bhushan",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Int J Infect Dis",
"key": "992_CR6",
"unstructured": "Bhushan BLS, Wanve S, Koradia P, Bhomia V, Soni P, Chakraborty S, et al. Efficacy and safety of pegylated interferon-α2b in moderate COVID-19: a phase 3, randomized, comparator-controlled, open-label study. Int J Infect Dis. 2021;111:281–7.",
"volume": "111",
"year": "2021"
},
{
"DOI": "10.1007/s12325-021-01998-y",
"author": "KY Chen",
"doi-asserted-by": "publisher",
"first-page": "910",
"journal-title": "Adv Ther",
"key": "992_CR7",
"unstructured": "Chen KY, Lee KY, Qin A, Luo CS, Yeh YK, Zheng JQ, et al. Clinical experience with ropeginterferon alfa-2b in the off-label use for the treatment of COVID-19 patients in Taiwan. Adv Ther. 2022;39:910–22.",
"volume": "39",
"year": "2022"
},
{
"key": "992_CR8",
"unstructured": "National Institutes of Health. COVID-19 treatment guidelines. Last Updated: March 6, 2023. Available on: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/"
},
{
"DOI": "10.1056/NEJMoa2209760",
"author": "G Reis",
"doi-asserted-by": "publisher",
"first-page": "518",
"journal-title": "N Engl J Med",
"key": "992_CR9",
"unstructured": "Reis G, Moreira Silva EAS, Medeiros Silva DC, Thabane L, Campos VHS, Ferreira TS, et al. Early treatment with pegylated interferon lambda for COVID-19. N Engl J Med. 2023;388:518–28.",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1128/JVI.01410-20",
"author": "KG Lokugamage",
"doi-asserted-by": "publisher",
"first-page": "e01410",
"journal-title": "J Virol",
"key": "992_CR10",
"unstructured": "Lokugamage KG, Hage A, deVries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94:e01410-e1420.",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104811",
"author": "E Mantlo",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "992_CR11",
"unstructured": "Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179: 104811.",
"volume": "179",
"year": "2020"
},
{
"DOI": "10.1007/s40261-021-01026-5",
"author": "N Miyachi",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "Clin Drug Investig",
"key": "992_CR12",
"unstructured": "Miyachi N, Zagrijtschuk O, Kang L, Yonezu K, Qin A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin Drug Investig. 2021;41:391–404.",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1111/bcp.15176",
"author": "YW Huang",
"doi-asserted-by": "publisher",
"first-page": "2396",
"journal-title": "Br J Clin Pharmacol",
"key": "992_CR13",
"unstructured": "Huang YW, Qin A, Fang J, Wang TF, Tsai CW, Lin KC, et al. Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics, and safety in a phase I clinical trial. Br J Clin Pharmacol. 2022;88:2396–407.",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1007/s12325-021-01863-y",
"author": "YW Huang",
"doi-asserted-by": "publisher",
"first-page": "4756",
"journal-title": "Adv Ther",
"key": "992_CR14",
"unstructured": "Huang YW, Tsai CY, Tsai CW, Wang W, Zhang JJ, Qin A, et al. Pharmacokinetics and pharmacodynamics of novel long-acting ropeginterferon alpha-2b in healthy Chinese subjects. Adv Ther. 2021;38:4756–70.",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.3390/v14061128",
"author": "YW Huang",
"doi-asserted-by": "publisher",
"first-page": "1128",
"journal-title": "Viruses",
"key": "992_CR15",
"unstructured": "Huang YW, Qin A, Tsai CY, Chen PJ. Novel pegylated interferon for the treatment of chronic viral hepatitis. Viruses. 2022;14:1128.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/jgh3.12825",
"author": "CY Chen",
"doi-asserted-by": "publisher",
"first-page": "782",
"journal-title": "JGH Open.",
"key": "992_CR16",
"unstructured": "Chen CY, Chuang WL, Qin A, Zhang WH, Zhu LY, Zhang GQ, et al. A phase 3 clinical trial validating the potency and safety of an innovative, extra-long-acting interferon in chronic hepatitis C. JGH Open. 2022;6:782–91.",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.3389/fonc.2023.1109866",
"author": "A Qin",
"doi-asserted-by": "publisher",
"first-page": "1109866",
"journal-title": "Front Oncol",
"key": "992_CR17",
"unstructured": "Qin A, Urbansky RW, Yu L, Ahmed T, Mascrenhas J. An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: an overview of previous and ongoing studies with perspectives on the future. Front Oncol. 2023;13:1109866.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.2217/fon-2022-1141",
"doi-asserted-by": "crossref",
"key": "992_CR18",
"unstructured": "Jin J, Qin A, Zhang L, Shen WH, Wang W, Zhang JJ, et al. A phase 2 trial to assess the efficacy and safety of ropeginterferon alfa-2b in Chinese patients with polycythemia vera. Future Oncol 2023;Online ahead of print."
},
{
"DOI": "10.2217/fon-2022-0596",
"author": "S Verstovsek",
"doi-asserted-by": "publisher",
"first-page": "2999",
"journal-title": "Future Oncol",
"key": "992_CR19",
"unstructured": "Verstovsek S, Komatsu N, Gill H, Jin J, Lee SE, Hou HA, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022;18:2999–3009.",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1002/jgh3.12613",
"author": "HH Lin",
"doi-asserted-by": "publisher",
"first-page": "929",
"journal-title": "JGH Open.",
"key": "992_CR20",
"unstructured": "Lin HH, Hsu SJ, Lu SN, Chuang WL, Hsu CW, Chien RN, et al. Ropeginterferon alfa-2b in patients with genotype 1 chronic hepatitis C: pharmacokinetics, safety, and preliminary efficacy. JGH Open. 2021;5:929–40.",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/j.jfma.2020.09.018",
"author": "SJ Hsu",
"doi-asserted-by": "publisher",
"first-page": "956",
"journal-title": "J Formos Med Assoc",
"key": "992_CR21",
"unstructured": "Hsu SJ, Yu ML, Su CW, Peng CY, Chien RN, Lin HH, et al. Ropeginterferon alfa-2b administered every two weeks for patients with genotype 2 chronic hepatitis C. J Formos Med Assoc. 2021;120:956–64.",
"volume": "120",
"year": "2021"
},
{
"DOI": "10.1007/s12072-020-10098-y",
"author": "YW Huang",
"doi-asserted-by": "publisher",
"first-page": "997",
"journal-title": "Hepatol Int",
"key": "992_CR22",
"unstructured": "Huang YW, Hsu CW, Lu SN, Yu ML, Su CW, Su WW, et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol Int. 2020;14:997–1008.",
"volume": "14",
"year": "2020"
},
{
"key": "992_CR23",
"unstructured": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020 Aug;20(8):e192-e197."
},
{
"DOI": "10.1016/j.rxeng.2020.11.001",
"doi-asserted-by": "crossref",
"key": "992_CR24",
"unstructured": "Martínez Chamorro E, Díez Tascón A, Ibáñez Sanz L, Ossaba Vélez S, Borruel Nacenta S. Radiologic diagnosis of patients with COVID-19. Radiologia (Engl Ed). 2021 Jan-Feb;63(1):56–73."
},
{
"DOI": "10.4269/ajtmh.21-0606",
"author": "S Abd-Elsalam",
"doi-asserted-by": "publisher",
"first-page": "886",
"journal-title": "Am J Trop Med Hyg",
"key": "992_CR25",
"unstructured": "Abd-Elsalam S, Salama M, Soliman S, Naguib AM, Ibrahim IS, Torky M, et al. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am J Trop Med Hyg. 2021;106:886–90. https://doi.org/10.4269/ajtmh.21-0606.",
"volume": "106",
"year": "2021"
},
{
"key": "992_CR26",
"unstructured": "Shih W, Aisner J. Statistical design and analysis of clinical trials: principles and methods. 1st ed. Chapman & Hall/CRC Biostatistics Series; 2015."
},
{
"DOI": "10.1201/9781315183084",
"doi-asserted-by": "crossref",
"key": "992_CR27",
"unstructured": "Chow SC, Shao J, Wang HS, Lokhnygina Y. Sample Size calculations in clinical research 3rd ed. Chapman & Hall/CRC Biostatistics Series; 2017."
},
{
"author": "WD Liu",
"first-page": "00039",
"issue": "24",
"journal-title": "J Microbiol Immunol Infect",
"key": "992_CR28",
"unstructured": "Liu WD, Wang JT, Shih MC, Chen KH, Huang ST, Huang CF, et al. Effect of early dexamethasone on outcomes of COVID-19: A quasi-experimental study using propensity score matching. J Microbiol Immunol Infect. 2024;S1684–1182(24):00039–42.",
"volume": "S1684–1182",
"year": "2024"
},
{
"DOI": "10.1038/s41422-022-00619-9",
"author": "D Bojkova",
"doi-asserted-by": "publisher",
"first-page": "319",
"journal-title": "Cell Res",
"key": "992_CR29",
"unstructured": "Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. Reduced interferon antagonism but similar drug sensitivity in omicron variant compared to delta variant of SARS-CoV-2 isolates. Cell Res. 2022;32:319–21.",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1038/s41421-022-00408-z",
"author": "D Bojkova",
"doi-asserted-by": "publisher",
"first-page": "42",
"journal-title": "Cell Discov.",
"key": "992_CR30",
"unstructured": "Bojkova D, Rothenburger T, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. SARS-CoV-2 omicron variant virus isolates are highly sensitive to interferon treatment. Cell Discov. 2022;8:42.",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.drup.2023.100986",
"author": "A Vitiello",
"doi-asserted-by": "publisher",
"journal-title": "Drug Resist Updat",
"key": "992_CR31",
"unstructured": "Vitiello A, Zovi A, Rezza G. New emerging SARS-CoV-2 variants and antiviral agents. Drug Resist Updat. 2023;70: 100986.",
"volume": "70",
"year": "2023"
},
{
"DOI": "10.1016/j.immuni.2019.03.025",
"author": "HM Lazear",
"doi-asserted-by": "publisher",
"first-page": "907",
"journal-title": "Immunity",
"key": "992_CR32",
"unstructured": "Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–23.",
"volume": "50",
"year": "2019"
},
{
"DOI": "10.1016/j.chom.2020.05.008",
"author": "A Park",
"doi-asserted-by": "publisher",
"first-page": "870",
"journal-title": "Cell Host Microbe",
"key": "992_CR33",
"unstructured": "Park A, Iwasaki A. Type I and type III interferons- induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–8.",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.648004",
"doi-asserted-by": "crossref",
"key": "992_CR34",
"unstructured": "Contoli M, Papi A, Tomassetti L, Rizzo P, Vieceli Dalla Sega F, Fortini F, et al. Blood interferon-α levels and severity, outcomes, and inflammatory profiles in hospitalized COVID-19 patients. Front Immunol 2021;12:648004."
},
{
"DOI": "10.1126/science.abc6027",
"author": "J Hadjadj",
"doi-asserted-by": "publisher",
"first-page": "718",
"journal-title": "Science",
"key": "992_CR35",
"unstructured": "Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30566-X",
"author": "JJ Feld",
"doi-asserted-by": "publisher",
"first-page": "498",
"journal-title": "Lancet Respir Med",
"key": "992_CR36",
"unstructured": "Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9:498–510.",
"volume": "9",
"year": "2021"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-024-00992-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Phase 3, Randomized, Controlled Trial Evaluating the Efficacy and Safety of Ropeginterferon Alfa-2b in Patients with Moderate COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
liu20