Clinical study of artesunate in the treatment of coronavirus disease 2019
et al., China Critical Care Medicine, doi:10.3760/cma.j.cn121430-20200312-00412, Apr 2020
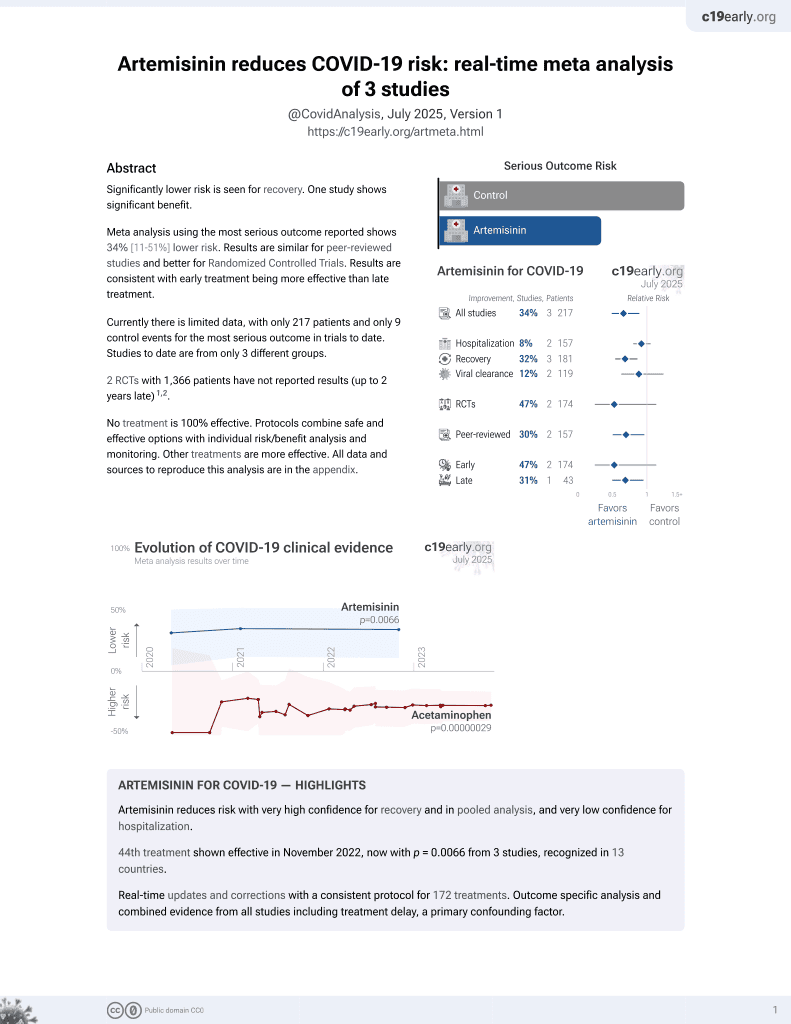
46th treatment shown to reduce risk in
November 2022, now with p = 0.0066 from 3 studies, recognized in 13 countries.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 43 hospitalized COVID-19 patients in China showing faster symptom improvement and shorter hospitalization with artesunate. Artesunate 60 mg twice daily for 10 days.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
recovery time, 31.2% lower, relative time 0.69, p = 0.02, treatment mean 3.33 (±1.91) n=18, control mean 4.84 (±2.19) n=25.
|
|
recovery time, 27.9% lower, relative time 0.72, p = 0.04, treatment mean 5.39 (±2.36) n=18, control mean 7.48 (±3.78) n=25, lung lesion absorption start.
|
|
recovery time, 17.2% lower, relative time 0.83, p = 0.03, treatment mean 14.11 (±4.16) n=18, control mean 17.04 (±4.42) n=25, lung lesion absorption greater than 70%.
|
|
hospitalization time, 8.2% lower, relative time 0.92, p = 0.22, treatment mean 16.56 (±3.71) n=18, control mean 18.04 (±3.97) n=25.
|
|
time to viral-, 29.3% lower, relative time 0.71, p = 0.05, treatment mean 4.72 (±2.16) n=18, control mean 6.68 (±3.76) n=25.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lin et al., 28 Apr 2020, retrospective, China, peer-reviewed, 9 authors.
, 有很好的应用前景。 【关键词】 青蒿琥酯 ; 新型冠状病毒肺炎 ; 治疗
doi:10.3760/cma.j.cn121430-20200312-00412
。治疗后青蒿琥酯联合治疗组 症状明显改善时间(d: 3.33±1.91 比 4.84±2.19) 、 2019-nCoV 核酸检测转阴时间(d: 4.72±2.16 比 6.68±3.76) 、 肺部病灶开始吸收时间(d : 5.39±2.36 比 7.48±3.78) 、病灶吸收>70% 时间(d : 14.11±4.16 比 17.04±4.42) 以及住院时间(d : 16.56±3.71 比 18
References
Cd, None, 临床医药文献电子杂志, doi:10.3877/j.issn.2095-8242.2018.87.166
Hu, Li, Zhang, Preparation of liposomal artesunate dry powder inhalers and the effect on the acute lung injury of rats [J], Acta Pharm Sin, doi:10.16438/j.0513-4870.2016-0848
Lang, Zhang, Fu, Clinical features and laboratory indicators in progression of corona virus disease 2019 to severe type, Chin J TCM WM Crit Care, doi:10.3969/j.issn.1008-9691.2020.01.007
Li, Fan, Li, Research progress on antiviral effect of artesunate [J/CD, J Clin Med Liter (Electronic Edition), doi:10.3877/j.issn.2095-8242.2018.87.166
国家卫生健康委员会, Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 3) [EB/OL, 中国中西医结合急救杂志, doi:10.3969/j.issn.1008-9691.2020.01.007
[1 ; Wang, Horby, Hayden, A novel coronavirus outbreak of global health concern, Lancet, doi:10.1016/S0140-6736(20)30185-9
[10 ; Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China [J], N Engl J Med, doi:10.1056/NEJMoa2002032
[5 ; Xu, Shi, Wang, Expert Panel of Critical Care Medicine for Corona Virus Disease 2019 in Shenzhen. The Shenzhen 2020 guidelines for the diagnosis and treatment of severe (severe/critical) coronavirus disease 2019, Chin J TCM WM Crit Care, doi:10.1016/S2213-2600(20)30076-X.[6]深圳市新型冠状病毒肺炎救治重症医学专家组
[7 ; Chou, Marousek, Auerochs, The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants [J], Antiviral Res, doi:10.1016/j.antiviral.2011.07.018
[9 ; Holshue, Debolt, Lindquist, First case of 2019 novel coronavirus in the United States [J], N Engl J Med, doi:10.1056/NEJMoa2001191