Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry
et al., Annals of Oncology, doi:10.1016/j.annonc.2021.01.067, Jan 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
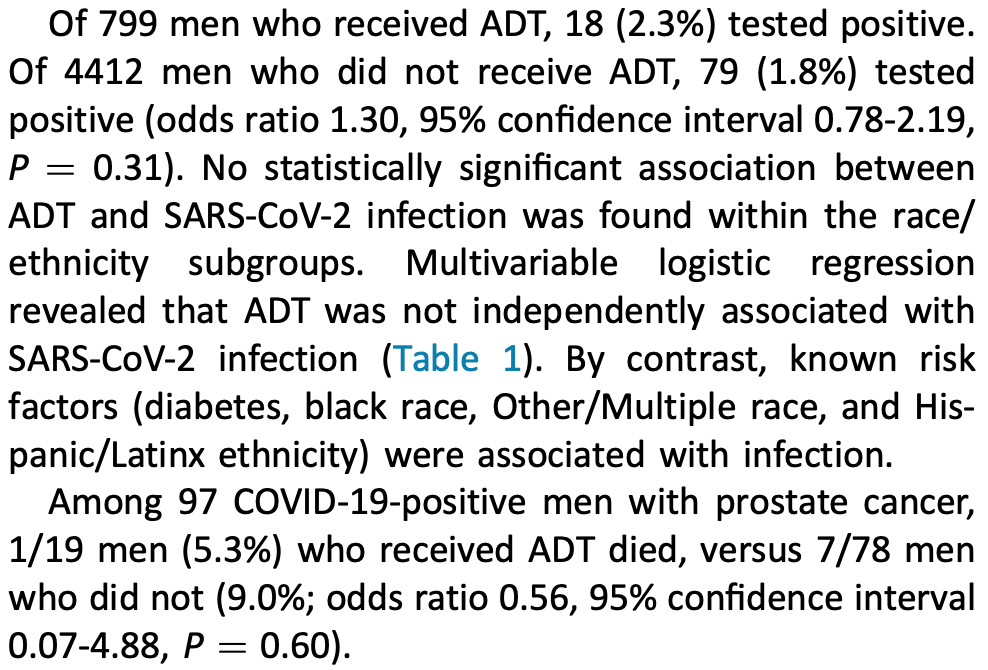
Retrospective 5,211 prostate cancer patients, 799 on ADT, showing no significant differences in COVID-19 outcomes with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 21.1% lower, RR 0.79, p = 1.00, treatment 1 of 799 (0.1%), control 7 of 4,412 (0.2%), NNT 2985.
|
|
risk of case, 17.6% higher, RR 1.18, p = 0.54, treatment 18 of 799 (2.3%), control 79 of 4,412 (1.8%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kwon et al., 29 Jan 2021, retrospective, USA, peer-reviewed, 7 authors.
Effectiveness of famotidine on the risk of poor prognosis in patients with COVID-19: A nationwide cohort study in Korea
Heliyon, doi:10.1016/j.heliyon.2023.e16171
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution Statement Rosie Kwon, Hyung Jun Kim,Seung Won Lee,: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Lee Smith, Ai Koyanagi: Contributed reagents, materials, analysis tools or data. Jae Il Shin, Tae-Jin Song, Dong Keon Yon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Conflicts of interest The authors declare no conflicts of interest.
Additional information No additional information is available for this paper. ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J o u r n a l P r e -p r o o f
References
Brennan, Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial, Gut, doi:10.1136/gutjnl-2022-326952
Cha, Jung, Seo, Rahmati, The emerging pandemic recent: SARS-CoV-2, doi:10.54724/lc.2023.e2
Chang, Woo, Park, Lee, Song, Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study, Eur J Prev Cardiol, doi:10.1177/2047487319886018
Freedberg, Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Hogan Ii, Dual-histamine receptor blockade with cetirizine -famotidine reduces pulmonary symptoms in COVID-19 patients, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2020.101942
Janowitz, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut, doi:10.1136/gutjnl-2020-321852
Kim, Kim, Kim, Kim, Short and Long-Term Mortality Trends for Cancer Patients with Septic Shock Stratified by Cancer Type from 2009 to 2017: A Population-Based Cohort Study, Cancers (Basel), doi:10.3390/cancers13040657
Kim, Yeniova, Global, regional, and national incidence and mortality of COVID-19 in 237 countries and territories, Life Cycle, doi:10.54724/lc.2022.e10
Kow, Abdul Sattar Burud, Hasan, Use of Famotidine and Risk of Severe Course of Illness in Patients With COVID-19: A Meta-analysis, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.03.001
Kritas, Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy, J Biol Regul Homeost Agents, doi:10.23812/20-Editorial-Kritas
Kuno, So, Takahashi, Egorova, The association between famotidine and in-hospital mortality of patients with COVID-19, J Med Virol, doi:10.1002/jmv.27375
Kwon, National trends in physical activity among adolescents in South Korea before and during the COVID-19 pandemic, 2009-2021, J Med Virol, doi:10.1002/jmv.28456
Lee, Acharya, Shin, Propensity score matching for causal inference and reducing the confounding effects: statistical standard and guideline of Life Cycle Committee, The Lancet. Rheumatology, doi:10.54724/lc.2022.e18
Lee, Association between mental illness and COVID-19 in South Korea: a post-hoc analysis. The lancet, Psychiatry, doi:10.1016/s2215-0366(21)00043-2
Lee, Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study, Lancet Psychiatry, doi:10.1016/s2215-0366(20)30421-1
Lee, Estimating COVID-19 Infection and Severity Risks in Patients with Chronic Rhinosinusitis: A Korean Nationwide Cohort Study. The journal of allergy and clinical immunology, doi:10.1016/j.jaip.2021.03.044
Lee, Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life Cycle Committee, Life Cycle, doi:10.54724/lc.2022.e1
Lee, Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study, British journal of sports medicine, doi:10.1136/bjsports-2021-104203
Lee, Proton pump inhibitors and the risk of severe COVID-19: a post-hoc analysis from the Korean nationwide cohort, Gut, doi:10.1136/gutjnl-2020-323672
Lee, Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee, Life Cycle, doi:10.54724/lc.2022.e3
Lee, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut, doi:10.1136/gutjnl-2020-322248
Lee, Yoon, Jang, Lee, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SMD, standardized mean difference; SD, standard deviation; COPD, chronic obstructive pulmonary disease, Thorax, doi:10.1136/thoraxjnl-2020-215322
Ma, Patel, Vemparala, Krishnamurthy, Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus, Scientific reports, doi:10.1038/s41598-022-09639-2
Malone, COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms. Res Sq, doi:10.21203/rs.3.rs-30934/v2
Mather, Seip, Mckay, Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19, Am J Gastroenterol, doi:10.14309/ajg.0000000000000832
Mukherjee, Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection, The Journal of biological chemistry, doi:10.1016/j.jbc.2021.100925
Mura, Real-world evidence for improved outcomes with histamine antagonists and aspirin in 22,560 COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00689-y
Pahwani, Efficacy of Oral Famotidine in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2, Cureus, doi:10.7759/cureus.22404
Reis, Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial, Lancet Regional Health. Americas, doi:10.1016/j.lana.2021.100142
Rommasi, Nasiri, Mirsaeidi, Immunomodulatory agents for COVID-19 treatment: possible mechanism of action and immunopathology features, Mol Cell Biochem, doi:10.1007/s11010-021-04325-9
Shin, Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity, Nature, doi:10.1038/s41586-020-2601-5
Shoaibi, Fortin, Weinstein, Berlin, Ryan, Comparative Effectiveness of Famotidine in Hospitalized COVID-19 Patients, Am J Gastroenterol, doi:10.14309/ajg.0000000000001153
Son, Lim, Park, Trend of Prevalence of Atrial Fibrillation and use of Oral Anticoagulation Therapy in Patients With Atrial Fibrillation in South Korea, J Epidemiol, doi:10.2188/jea.JE20160149
Sun, Does Famotidine Reduce the Risk of Progression to Severe Disease, Death, and Intubation for COVID-19 Patients? A Systemic Review and Meta-Analysis, Dig Dis Sci, doi:10.1007/s10620-021-06872-z
Wen, Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis, Ann Med, doi:10.1080/07853890.2022.2034936
Woo, Lee, Kim, Effect of pioglitazone in acute ischemic stroke patients with diabetes mellitus: a nested case-control study, Cardiovasc Diabetol, doi:10.1186/s12933-019-0874-5
Wu, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B, doi:10.1016/j.apsb.2020.02.008
Xu, A meta-analysis on the risk factors adjusted association between cardiovascular disease and COVID-19 severity, BMC public health, doi:10.1186/s12889-021-11051-w
Yeramaneni, Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus J, doi:10.1053/j.gastro.2020.10.011
Yoo, Marshall, Cho, Yoo, Lee, N-Nitrosodimethylamine-contaminated ranitidine and risk of cancer in South Korea: a nationwide cohort study, Life Cycle, doi:10.54724/lc.2021.e1
DOI record:
{
"DOI": "10.1016/j.annonc.2021.01.067",
"ISSN": [
"0923-7534"
],
"URL": "http://dx.doi.org/10.1016/j.annonc.2021.01.067",
"alternative-id": [
"S0923753421000958"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Annals of Oncology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.annonc.2021.01.067"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Kwon",
"given": "D.H.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Vashisht",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borno",
"given": "H.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aggarwal",
"given": "R.R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Small",
"given": "E.J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butte",
"given": "A.J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "F.W.",
"sequence": "additional"
}
],
"container-title": [
"Annals of Oncology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"annalsofoncology.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
9
]
],
"date-time": "2021-02-09T13:09:05Z",
"timestamp": 1612876145000
},
"deposited": {
"date-parts": [
[
2021,
12,
5
]
],
"date-time": "2021-12-05T07:13:53Z",
"timestamp": 1638688433000
},
"indexed": {
"date-parts": [
[
2021,
12,
18
]
],
"date-time": "2021-12-18T00:30:10Z",
"timestamp": 1639787410549
},
"is-referenced-by-count": 4,
"issn-type": [
{
"type": "print",
"value": "0923-7534"
}
],
"issue": "5",
"issued": {
"date-parts": [
[
2021,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0923753421000958?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0923753421000958?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "678-679",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
5
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli",
"doi-asserted-by": "crossref",
"first-page": "1040",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "10.1016/j.annonc.2021.01.067_bib1",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.015",
"article-title": "Androgen deprivation and SARS-CoV-2 in men with prostate cancer",
"author": "Koskinen",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "10.1016/j.annonc.2021.01.067_bib2",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.stem.2020.11.009",
"article-title": "Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men",
"author": "Samuel",
"doi-asserted-by": "crossref",
"first-page": "876",
"issue": "6",
"journal-title": "Cell Stem Cell",
"key": "10.1016/j.annonc.2021.01.067_bib3",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"article-title": "Identification of antiviral antihistamines for COVID-19 repurposing",
"author": "Reznikov",
"doi-asserted-by": "crossref",
"first-page": "173",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.annonc.2021.01.067_bib4",
"volume": "538",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "1419",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "10.1016/j.annonc.2021.01.067_bib5",
"volume": "31",
"year": "2020"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"score": 1,
"short-container-title": [
"Annals of Oncology"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Oncology",
"Hematology"
],
"subtitle": [],
"title": [
"Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "32"
}
