Efficacy of Oral Famotidine in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2
et al., Cureus, doi:10.7759/cureus.22404, Feb 2022
Famotidine for COVID-19
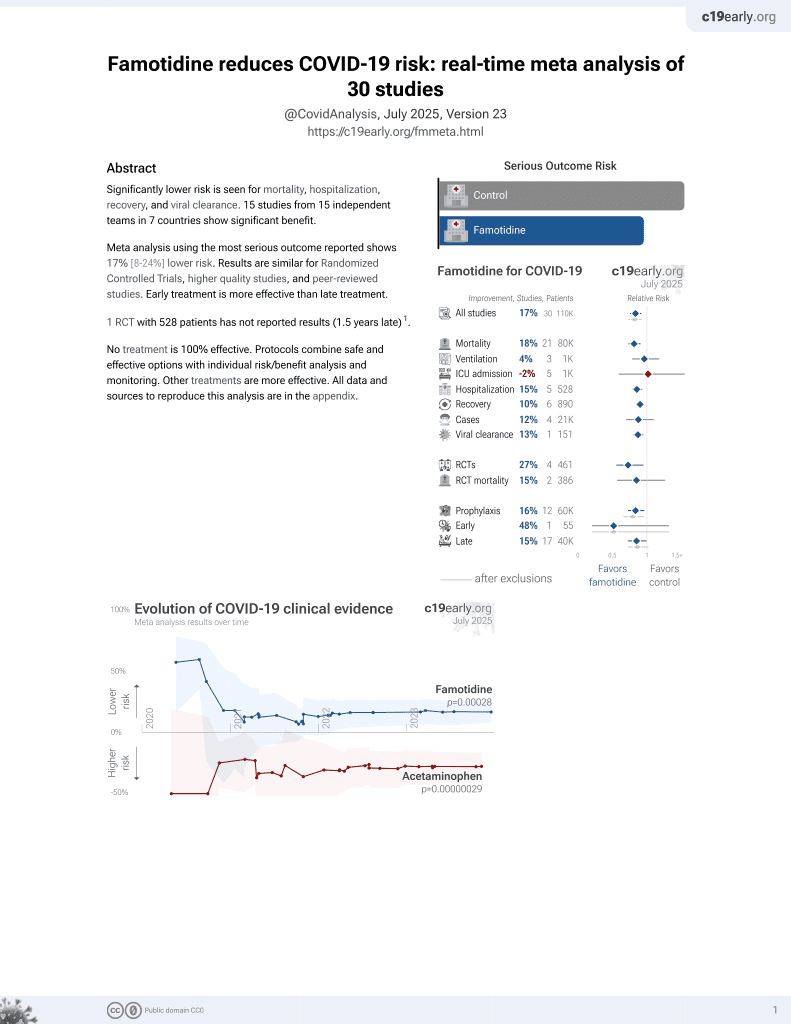
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
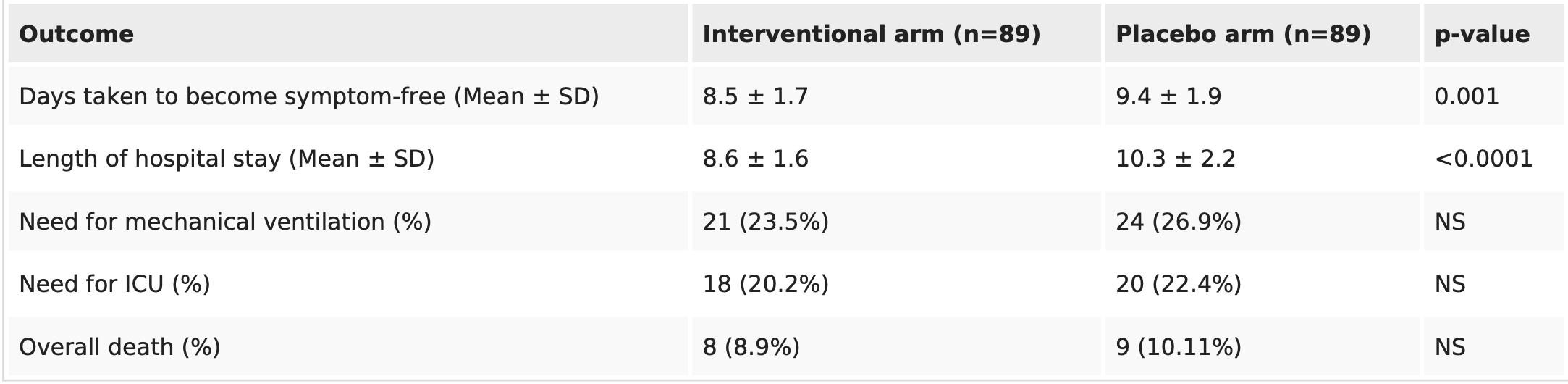
RCT with 89 famotidine and 89 control patients in Pakistan, showing faster recovery but no significant difference in mortality. 40mg oral famotidine daily.
|
risk of death, 11.1% lower, RR 0.89, p = 1.00, treatment 8 of 89 (9.0%), control 9 of 89 (10.1%), NNT 89.
|
|
risk of mechanical ventilation, 12.5% lower, RR 0.88, p = 0.73, treatment 21 of 89 (23.6%), control 24 of 89 (27.0%), NNT 30.
|
|
risk of ICU admission, 10.0% lower, RR 0.90, p = 0.86, treatment 18 of 89 (20.2%), control 20 of 89 (22.5%), NNT 44.
|
|
hospitalization time, 16.5% lower, relative time 0.83, p < 0.001, treatment mean 8.6 (±1.6) n=89, control mean 10.3 (±2.2) n=89.
|
|
recovery time, 9.6% lower, relative time 0.90, p = 0.001, treatment mean 8.5 (±1.7) n=89, control mean 9.4 (±1.9) n=89.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pahwani et al., 20 Feb 2022, Randomized Controlled Trial, Pakistan, peer-reviewed, mean age 52.0, 8 authors, study period December 2020 - September 2021.
Efficacy of Oral Famotidine in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2
Cureus, doi:10.7759/cureus.22404
Introduction The clinical benefit of famotidine has been observed in the management of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, its use in the management of SARS-CoV-2 is intriguing and not well established yet. In this study, we aimed to determine the role of famotidine as adjuvant therapy in improving the outcome of patients hospitalized with coronavirus disease-2019 .
Methods This two-arm open-label randomized interventional study was conducted in the COVID-19 unit of a tertiary care hospital in Pakistan from December 2020 to September 2021. Patients between the ages of 18 to 65 years, hospitalized with COVID-19 infection, were enrolled in the study. Participants were randomized into two groups. The intervention group received 40 mg oral famotidine daily in addition to the standard care and the control group received standard care as per national guidelines for the treatment of COVID-19 in Pakistan.
Results Patients admitted with COVID-19 who received famotidine took comparatively fewer days to become symptom-free (8.5 ± 1.7 vs. 9.4 ± 1.9 days, p-value: <0.001) and spent fewer days in hospital (8.6 ± 1.6 vs. 10.3 ± 2.2 days; p-value: <0.0001). However, the overall difference in the need for mechanical ventilation and mortality between the interventional arm and placebo was not significant.
Conclusion In this study, adding famotidine to standard treatment of COVID-19 was associated with faster clinical recovery and shorter stay in the hospital. However, there was no difference in the need for mechanical ventilation, need for intensive care unit, and overall mortality. Further large-scale studies are needed to understand the role of famotidine in COVID-19 and its mechanism of action in patients with COVID-19.
Conclusions In this study, adding famotidine to standard treatment of COVID-19 was associated with faster clinical recovery and shorter hospital stay. However, there was no difference in the need for mechanical ventilation, the need for ICU, and overall mortality. Further large-scale studies are needed to understand the role of famotidine in COVID-19 and its possible mechanism of action in patients with COVID-19.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Jinnah Sindh Medical University Ethics Committee issued approval JSMU/IRB/2020/12. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Ai, Yang, Hou, Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases, Radiology, doi:10.1148/radiol.2020200642
Alonso, Zappia, Cabrera, Davio, Shayo et al., Physiological implications of biased signaling at histamine H2 receptors, Front Pharmacol, doi:10.3389/fphar.2015.00045
Balouch, Vontela, Yeakel, Alnouri, Sataloff, Role of famotidine and other acid reflux medications for SARS-CoV-2: a pilot study, doi:10.1016/j.jvoice.2021.01.007
Chakraborty, Sharma, Sharma, Bhattacharya, Lee, SARS-CoV-2 causing pneumoniaassociated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options, Eur Rev Med Pharmacol Sci, doi:10.26355/eurrev_202004_20871
Chan, Yip, To, Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens, J Clin Microbiol, doi:10.1128/JCM.00310-20
Chen, Qi, Liu, Clinical progression of patients with COVID-19 in Shanghai, China, J Infect, doi:10.1016/j.jinf.2020.03.004
Freedberg, Conigliaro, Wang, Tracey, Callahan et al., Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Krystel-Whittemore, Dileepan, Wood, Mast cell: a multi-functional master cell, Front Immunol, doi:10.3389/fimmu.2015.00620
Lei, Zhang, Yu, Patlas, COVID-19 infection: early lessons, Can Assoc Radiol J, doi:10.1177/0846537120914428
Lorenzo, Fernández-Hernando, Cirino, Sessa, Akt1 is critical for acute inflammation and histamine-mediated vascular leakage, Proc Natl Acad Sci U S A, doi:10.1073/pnas.0904073106
Luo, Chen, Zhao, Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function, Basic Res Cardiol, doi:10.1007/s00395-013-0342-4
Malone, Tisdall, Smith, COVID-19: famotidine, histamine, mast cells, and mechanisms, doi:10.21203/rs.3.rs-30934/v2
Mather, Seip, Mckay, Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19, Am J Gastroenterol, doi:10.14309/ajg.0000000000000832
Pahwani, None, Cureus, doi:10.7759/cureus.224043of4
Pahwani, None, Cureus, doi:10.7759/cureus.224044of4
Salehi, Abedi, Balakrishnan, Gholamrezanezhad, Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients, Am J Roentgenol, doi:10.2214/AJR.20.23034
Sun, Chen, Hu, Does famotidine reduce the risk of progression to severe disease, death, and intubation for COVID-19 patients? A systemic review and meta-analysis, Dig Dis Sci, doi:10.1007/s10620-021-06872-z
DOI record:
{
"DOI": "10.7759/cureus.22404",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.22404",
"author": [
{
"affiliation": [],
"family": "Pahwani",
"given": "Suraksha",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jadwani",
"given": "Mahesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhanwani",
"given": "Aperna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gul",
"given": "Mehak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lal",
"given": "Darshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rakesh",
"given": "FNU",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shabbir",
"given": "Raffey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizwan",
"given": "Amber",
"sequence": "additional"
}
],
"container-title": [
"Cureus"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
20
]
],
"date-time": "2022-02-20T18:27:14Z",
"timestamp": 1645381634000
},
"deposited": {
"date-parts": [
[
2022,
2,
20
]
],
"date-time": "2022-02-20T18:27:21Z",
"timestamp": 1645381641000
},
"indexed": {
"date-parts": [
[
2022,
2,
20
]
],
"date-time": "2022-02-20T18:41:19Z",
"timestamp": 1645382479144
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2168-8184"
}
],
"issued": {
"date-parts": [
[
2022,
2,
20
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/78980-efficacy-of-oral-famotidine-in-patients-hospitalized-with-severe-acute-respiratory-syndrome-coronavirus-2",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4492",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2022,
2,
20
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
20
]
]
},
"publisher": "Cureus, Inc.",
"reference": [
{
"DOI": "10.26355/eurrev_202004_20871",
"article-title": "SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options",
"author": "Chakraborty C",
"doi-asserted-by": "publisher",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "ref1",
"unstructured": "Chakraborty C, Sharma AR, Sharma G, Bhattacharya M, Lee SS. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020, 24:4016-26. 10.26355/eurrev_202004_20871",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.2214/AJR.20.23034",
"article-title": "Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients",
"author": "Salehi S",
"doi-asserted-by": "publisher",
"journal-title": "Am J Roentgenol",
"key": "ref2",
"unstructured": "Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020, 215:87-93. 10.2214/AJR.20.23034",
"volume": "215",
"year": "2020"
},
{
"DOI": "10.1128/JCM.00310-20",
"article-title": "Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens",
"author": "Chan JF",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Microbiol",
"key": "ref3",
"unstructured": "Chan JF, Yip CC, To KK, et al.. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020, 58:e00310-20. 10.1128/JCM.00310-20",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.004",
"article-title": "Clinical progression of patients with COVID-19 in Shanghai, China",
"author": "Chen J",
"doi-asserted-by": "publisher",
"journal-title": "J Infect",
"key": "ref4",
"unstructured": "Chen J, Qi T, Liu L, et al.. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020, 80:e1-6. 10.1016/j.jinf.2020.03.004",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang C",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref5",
"unstructured": "Huang C, Wang Y, Li X, et al.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020, 395:497-506. 10.1016/S0140-6736(20)30183-5",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200642",
"article-title": "Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases",
"author": "Ai T",
"doi-asserted-by": "publisher",
"journal-title": "Radiology",
"key": "ref6",
"unstructured": "Ai T, Yang Z, Hou H, et al.. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020, 296:e32-40. 10.1148/radiol.2020200642",
"volume": "296",
"year": "2020"
},
{
"DOI": "10.1177/0846537120914428",
"article-title": "COVID-19 infection: early lessons",
"author": "Lei Y",
"doi-asserted-by": "publisher",
"journal-title": "Can Assoc Radiol J",
"key": "ref7",
"unstructured": "Lei Y, Zhang HW, Yu J, Patlas MN. COVID-19 infection: early lessons. Can Assoc Radiol J. 2020, 71:251-2. 10.1177/0846537120914428",
"volume": "71",
"year": "2020"
},
{
"key": "ref8",
"unstructured": "Clinical management summary. (2022). Accessed. February 1, 2022: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/clinical-management-summary/."
},
{
"DOI": "10.1016/j.jvoice.2021.01.007",
"article-title": "Role of famotidine and other acid reflux medications for SARS-CoV-2: a pilot study (IN PRESS)",
"author": "Balouch B",
"doi-asserted-by": "publisher",
"journal-title": "J Voice",
"key": "ref9",
"unstructured": "Balouch B, Vontela S, Yeakel H, Alnouri G, Sataloff RT. Role of famotidine and other acid reflux medications for SARS-CoV-2: a pilot study (IN PRESS). J Voice. 2021, 10.1016/j.jvoice.2021.01.007",
"year": "2021"
},
{
"DOI": "10.14309/ajg.0000000000000832",
"article-title": "Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19",
"author": "Mather JF",
"doi-asserted-by": "publisher",
"journal-title": "Am J Gastroenterol",
"key": "ref10",
"unstructured": "Mather JF, Seip RL, McKay RG. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am J Gastroenterol. 2020, 115:1617-23. 10.14309/ajg.0000000000000832",
"volume": "115",
"year": "2020"
},
{
"key": "ref11",
"unstructured": "Sample size calculations. (2018). Accessed. February 17, 2022: https://epitools.ausvet.com.au/samplesize."
},
{
"key": "ref12",
"unstructured": "Research randomizer. (2013). Accessed. February 17, 2022: https://www.randomizer.org/."
},
{
"DOI": "10.21203/rs.3.rs-30934/v2",
"article-title": "COVID- 19: famotidine, histamine, mast cells, and mechanisms [PREPRINT]",
"author": "Malone RW",
"doi-asserted-by": "publisher",
"journal-title": "Res Sq",
"key": "ref13",
"unstructured": "Malone RW, Tisdall P, Fremont-Smith P, et al.. COVID- 19: famotidine, histamine, mast cells, and mechanisms [PREPRINT]. Res Sq. 2020, 10.21203/rs.3.rs-30934/v2",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"article-title": "Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study",
"author": "Freedberg DE",
"doi-asserted-by": "publisher",
"journal-title": "Gastroenterology",
"key": "ref14",
"unstructured": "Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020, 159:1129-31. 10.1053/j.gastro.2020.05.053",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1007/s10620-021-06872-z",
"article-title": "Does famotidine reduce the risk of progression to severe disease, death, and intubation for COVID-19 patients? A systemic review and meta-analysis",
"author": "Sun C",
"doi-asserted-by": "publisher",
"journal-title": "Dig Dis Sci",
"key": "ref15",
"unstructured": "Sun C, Chen Y, Hu L, et al.. Does famotidine reduce the risk of progression to severe disease, death, and intubation for COVID-19 patients? A systemic review and meta-analysis. Dig Dis Sci. 2021, 66:3929-37. 10.1007/s10620-021-06872-z",
"volume": "66",
"year": "2021"
},
{
"key": "ref16",
"unstructured": "New York clinical trial quietly tests heartburn remedy against coronavirus. (2020). Accessed. January 31, 2022: https://legacy.pulitzercenter.org/reporting/new-york-clinical-trial-quietly-tests-heartburn-remedy-against-coronavirus."
},
{
"DOI": "10.1007/s00395-013-0342-4",
"article-title": "Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function",
"author": "Luo T",
"doi-asserted-by": "publisher",
"journal-title": "Basic Res Cardiol",
"key": "ref17",
"unstructured": "Luo T, Chen B, Zhao Z, et al.. Histamine H2 receptor activation exacerbates myocardial ischemia/reperfusion injury by disturbing mitochondrial and endothelial function. Basic Res Cardiol. 2013, 108:342. 10.1007/s00395-013-0342-4",
"volume": "108",
"year": "2013"
},
{
"DOI": "10.1073/pnas.0904073106",
"article-title": "Akt1 is critical for acute inflammation and histamine-mediated vascular leakage",
"author": "Di Lorenzo A",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "ref18",
"unstructured": "Di Lorenzo A, Fernández-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009, 106:14552-7. 10.1073/pnas.0904073106",
"volume": "106",
"year": "2009"
},
{
"DOI": "10.3389/fimmu.2015.00620",
"article-title": "Mast cell: a multi-functional master cell",
"author": "Krystel-Whittemore M",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ref19",
"unstructured": "Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2016, 6:620. 10.3389/fimmu.2015.00620",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.3389/fphar.2015.00045",
"article-title": "Physiological implications of biased signaling at histamine H2 receptors",
"author": "Alonso N",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "ref20",
"unstructured": "Alonso N, Zappia CD, Cabrera M, Davio CA, Shayo C, Monczor F, Fernández NC. Physiological implications of biased signaling at histamine H2 receptors. Front Pharmacol. 2015, 6:45. 10.3389/fphar.2015.00045",
"volume": "6",
"year": "2015"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": [
"Efficacy of Oral Famotidine in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2"
],
"type": "journal-article"
}