Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19
et al., American Journal of Gastroenterology, doi:10.14309/ajg.0000000000000832, Aug 2020
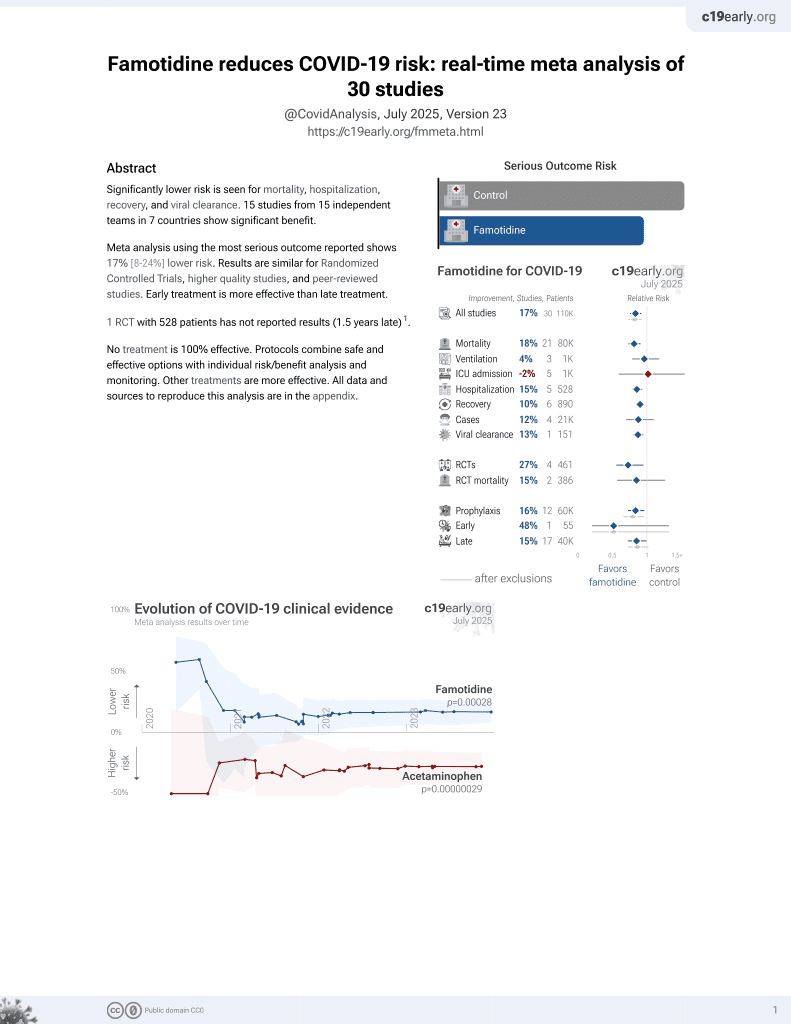
Famotidine for COVID-19
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
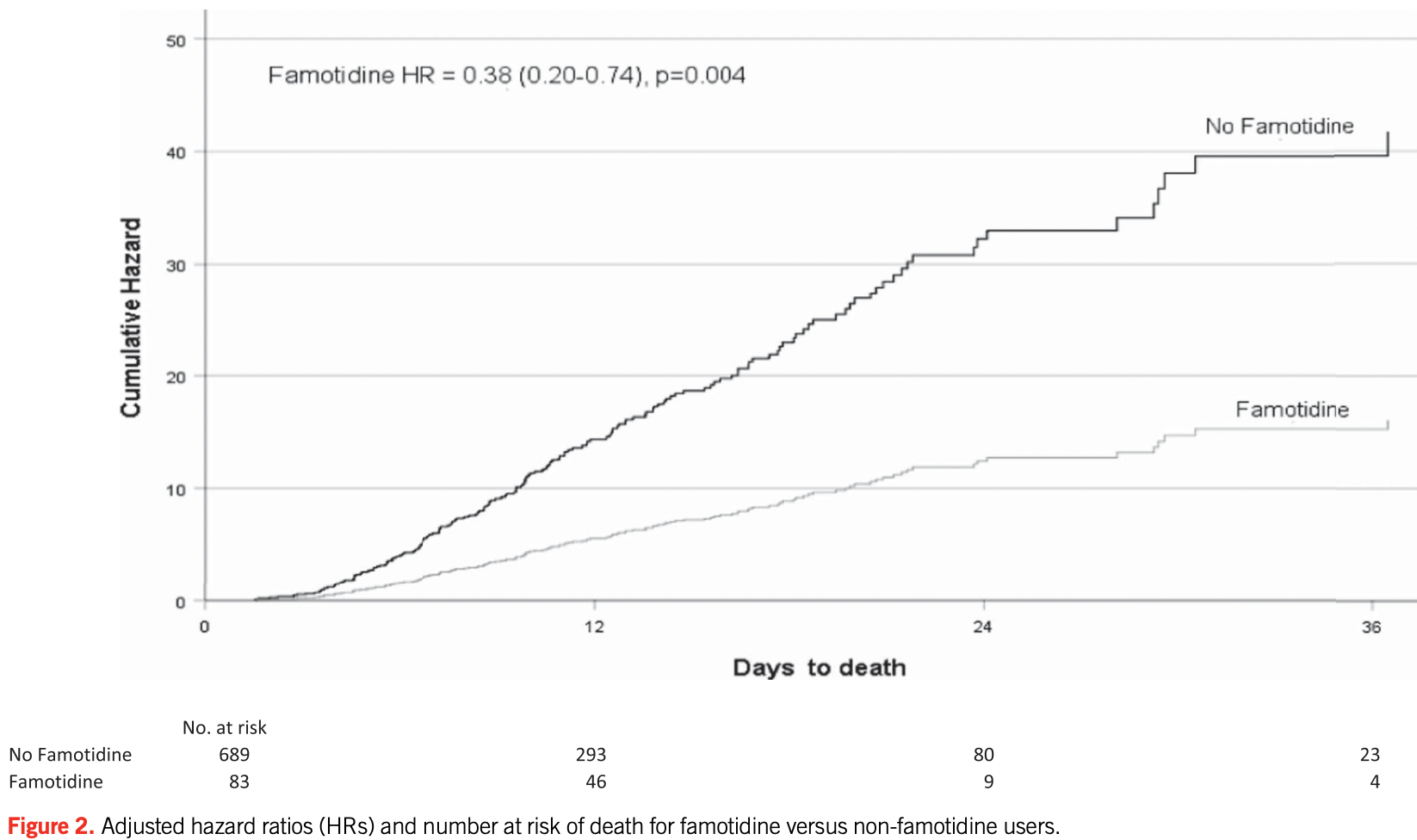
PSM retrospective 878 hospitalized patients in the USA, 83 with existing famotidine use, showing significantly lower mortality with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 61.4% lower, HR 0.39, p = 0.004, treatment 83, control 689, propensity score matching, Cox proportional hazards.
|
|
risk of death/intubation, 50.5% lower, HR 0.49, p = 0.003, treatment 83, control 689, propensity score matching, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mather et al., 26 Aug 2020, retrospective, USA, peer-reviewed, 3 authors.
Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19
American Journal of Gastroenterology, doi:10.14309/ajg.0000000000000832
INTRODUCTION: To compare outcomes in patients hospitalized with coronavirus (COVID-19) receiving famotidine therapy with those not receiving famotidine.
Study Highlights WHAT IS KNOWN? 3 Despite multiple trials that are currently underway to investigate the safety and efficacy of a large number of possible therapeutic agents, no drug to date has been shown to reduce COVID-19 mortality. 3 It has been postulated that famotidine's effect is achieved via its antagonism or inverse agonism of the histamine-2 receptor, inferring that the SARS-CoV-2 infection that results in COVID-19 is at least partially mediated by pathological histamine release.
COVID-19 Mather et al.
References
Anand, Ziebuhr, Wadhwani, Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs, Science
Borrell, New York clinical trial quietly tests heartburn remedy against coronavirus
Bourinbaiar, Fruhstorfer, The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: Identification of a new class of antiviral agents, Life Sci
Burde, Seifert, Buschauer, Histamine inhibits activation of human neutrophils and HL-60 leukemic cells via H2-receptors, Naunyn Schmiedebergs Arch Pharmacol
Caughey, Raymond, Wolters, Angiotensin II generation by mast cell alpha-and beta-chymases, Biochim Biophys Acta
Eliezer, Hautefort, Hamel, Sudden and complete olfactory loss of function as a possible symptom of COVID-19, JAMA Otolaryngol Head Neck Surg
Ezeamuzie, Philips, Histamine, 2) receptors mediate the inhibitory effect of histamine on human eosinophil degranulation, Br J Pharmacol
Flamand, Plante, Picard, Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: Involvement of the H2 receptor and cAMP, Br J Pharmacol
Freedberg, Conigliaro, Wang, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: A propensity score matched retrospective cohort study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Giacomelli, Pezzati, Conti, Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study, Clin Infect Dis
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: A case series, Gut
Kritas, Ronconi, Caraffa, Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy, J Biol Regul Homeost Agents
Malone, Tisdall, Smith, COVID-19: famotidine, histamine, mast cells, and mechanisms, Preprint Res Sq, doi:10.21203/rs.3.rs-30934/v2
Marshall, Portales-Cervantes, Leong, Mast cell responses to viruses and pathogen products, Int J Mol Sci
Metcalfe, Baram, Mekori, Mast cells, Physiol Rev
Mukai, Tsai, Saito, Mast cells as sources of cytokines, chemokines, and growth factors, Immunol Rev
Rabier, Damon, Chanez, Inhibition by histamine of plateletactivating-factor-induced neutrophil chemotaxis in bronchial asthma, Int Arch Allergy Appl Immunol
Theoharides, Alysandratos, Angelidou, Mast cells and inflammation, Biochim Biophys Acta
Tian, Hu, Niu, Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer, J Thorac Oncol
Wadee, Anderson, Sher, In vitro effects of histamine on migration, Int Arch Allergy Appl Immunol
Wu, Liu, Yang, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B
DOI record:
{
"DOI": "10.14309/ajg.0000000000000832",
"ISSN": [
"0002-9270",
"1572-0241"
],
"URL": "http://dx.doi.org/10.14309/ajg.0000000000000832",
"author": [
{
"affiliation": [],
"family": "Mather",
"given": "Jeffrey F.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Seip",
"given": "Richard L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKay",
"given": "Raymond G.",
"sequence": "additional"
}
],
"container-title": [
"American Journal of Gastroenterology"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
8,
26
]
],
"date-time": "2020-08-26T21:12:33Z",
"timestamp": 1598476353000
},
"deposited": {
"date-parts": [
[
2021,
5,
20
]
],
"date-time": "2021-05-20T04:01:20Z",
"timestamp": 1621483280000
},
"indexed": {
"date-parts": [
[
2022,
1,
19
]
],
"date-time": "2022-01-19T16:48:48Z",
"timestamp": 1642610928169
},
"is-referenced-by-count": 55,
"issn-type": [
{
"type": "print",
"value": "0002-9270"
},
{
"type": "electronic",
"value": "1572-0241"
}
],
"issue": "10",
"issued": {
"date-parts": [
[
2020,
8,
26
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2020
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.14309/ajg.0000000000000832",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1617-1623",
"prefix": "10.14309",
"published": {
"date-parts": [
[
2020,
8,
26
]
]
},
"published-online": {
"date-parts": [
[
2020,
8,
26
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1053/j.gastro.2020.05.053",
"article-title": "Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: A propensity score matched retrospective cohort study",
"author": "Freedberg",
"doi-asserted-by": "crossref",
"journal-title": "Gastroenterology",
"key": "R2-20210520",
"year": "2020"
},
{
"article-title": "Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: A case series",
"author": "Janowitz",
"first-page": "1",
"journal-title": "Gut",
"key": "R3-20210520",
"volume": "0",
"year": "2020"
},
{
"DOI": "10.1016/S0024-3205(96)00553-X",
"article-title": "The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: Identification of a new class of antiviral agents",
"author": "Bourinbaiar",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "23",
"journal-title": "Life Sci",
"key": "R4-20210520",
"volume": "59",
"year": "1996"
},
{
"article-title": "COVID-19: famotidine, histamine, mast cells, and mechanisms",
"author": "Malone",
"journal-title": "Preprint Res Sq",
"key": "R5-20210520",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"article-title": "Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "766",
"issue": "5",
"journal-title": "Acta Pharm Sin B",
"key": "R6-20210520",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1126/science.1085658",
"article-title": "Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "1763",
"issue": "5626",
"journal-title": "Science",
"key": "R7-20210520",
"volume": "300",
"year": "2003"
},
{
"article-title": "Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy",
"author": "Kritas",
"first-page": "9",
"issue": "1",
"journal-title": "J Biol Regul Homeost Agents",
"key": "R8-20210520",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa330",
"article-title": "Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study",
"author": "Giacomelli",
"doi-asserted-by": "crossref",
"first-page": "889",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "R9-20210520",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1001/jamaoto.2020.0832",
"article-title": "Sudden and complete olfactory loss of function as a possible symptom of COVID-19",
"author": "Eliezer",
"doi-asserted-by": "crossref",
"first-page": "674",
"journal-title": "JAMA Otolaryngol Head Neck Surg",
"key": "R10-20210520",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.jtho.2020.02.010",
"article-title": "Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "700",
"issue": "5",
"journal-title": "J Thorac Oncol",
"key": "R11-20210520",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1007/BF00717743",
"article-title": "Histamine inhibits activation of human neutrophils and HL-60 leukemic cells via H2-receptors",
"author": "Burde",
"doi-asserted-by": "crossref",
"first-page": "671",
"issue": "6",
"journal-title": "Naunyn Schmiedebergs Arch Pharmacol",
"key": "R12-20210520",
"volume": "340",
"year": "1989"
},
{
"DOI": "10.1159/000234967",
"article-title": "Inhibition by histamine of platelet-activating-factor-induced neutrophil chemotaxis in bronchial asthma",
"author": "Rabier",
"doi-asserted-by": "crossref",
"first-page": "314",
"issue": "2-3",
"journal-title": "Int Arch Allergy Appl Immunol",
"key": "R13-20210520",
"volume": "89",
"year": "1989"
},
{
"DOI": "10.1038/sj.bjp.0705654",
"article-title": "Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: Involvement of the H2 receptor and cAMP",
"author": "Flamand",
"doi-asserted-by": "crossref",
"first-page": "552",
"issue": "4",
"journal-title": "Br J Pharmacol",
"key": "R14-20210520",
"volume": "141",
"year": "2004"
},
{
"DOI": "10.1038/sj.bjp.0703556",
"article-title": "(2) receptors mediate the inhibitory effect of histamine on human eosinophil degranulation",
"author": "Ezeamuzie",
"doi-asserted-by": "crossref",
"first-page": "482",
"issue": "3",
"journal-title": "Br J Pharmacol",
"key": "R15-20210520",
"volume": "131",
"year": "2000"
},
{
"DOI": "10.1159/000232643",
"article-title": "In vitro effects of histamine on eosinophil migration",
"author": "Wadee",
"doi-asserted-by": "crossref",
"first-page": "322",
"issue": "3",
"journal-title": "Int Arch Allergy Appl Immunol",
"key": "R16-20210520",
"volume": "63",
"year": "1980"
},
{
"DOI": "10.1152/physrev.1997.77.4.1033",
"article-title": "Mast cells",
"author": "Metcalfe",
"doi-asserted-by": "crossref",
"first-page": "1033",
"issue": "4",
"journal-title": "Physiol Rev",
"key": "R17-20210520",
"volume": "77",
"year": "1997"
},
{
"DOI": "10.1111/imr.12634",
"article-title": "Mast cells as sources of cytokines, chemokines, and growth factors",
"author": "Mukai",
"doi-asserted-by": "crossref",
"first-page": "121",
"issue": "1",
"journal-title": "Immunol Rev",
"key": "R18-20210520",
"volume": "282",
"year": "2018"
},
{
"DOI": "10.1016/j.bbadis.2010.12.014",
"article-title": "Mast cells and inflammation",
"author": "Theoharides",
"doi-asserted-by": "crossref",
"first-page": "21",
"issue": "1",
"journal-title": "Biochim Biophys Acta",
"key": "R19-20210520",
"volume": "1822",
"year": "2012"
},
{
"DOI": "10.3390/ijms20174241",
"article-title": "Mast cell responses to viruses and pathogen products",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "4241",
"issue": "17",
"journal-title": "Int J Mol Sci",
"key": "R20-20210520",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1016/S0167-4838(00)00076-5",
"article-title": "Angiotensin II generation by mast cell alpha- and beta-chymases",
"author": "Caughey",
"doi-asserted-by": "crossref",
"first-page": "245",
"issue": "1-2",
"journal-title": "Biochim Biophys Acta",
"key": "R21-20210520",
"volume": "1480",
"year": "2000"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"score": 1,
"short-container-title": [
"Am J Gastroenterol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology",
"Hepatology"
],
"subtitle": [],
"title": [
"Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19"
],
"type": "journal-article",
"volume": "115"
}