Role of Famotidine and Other Acid Reflux Medications for SARS-CoV-2: A Pilot Study
et al., Journal of Voice, doi:10.1016/j.jvoice.2021.01.007, Jan 2021
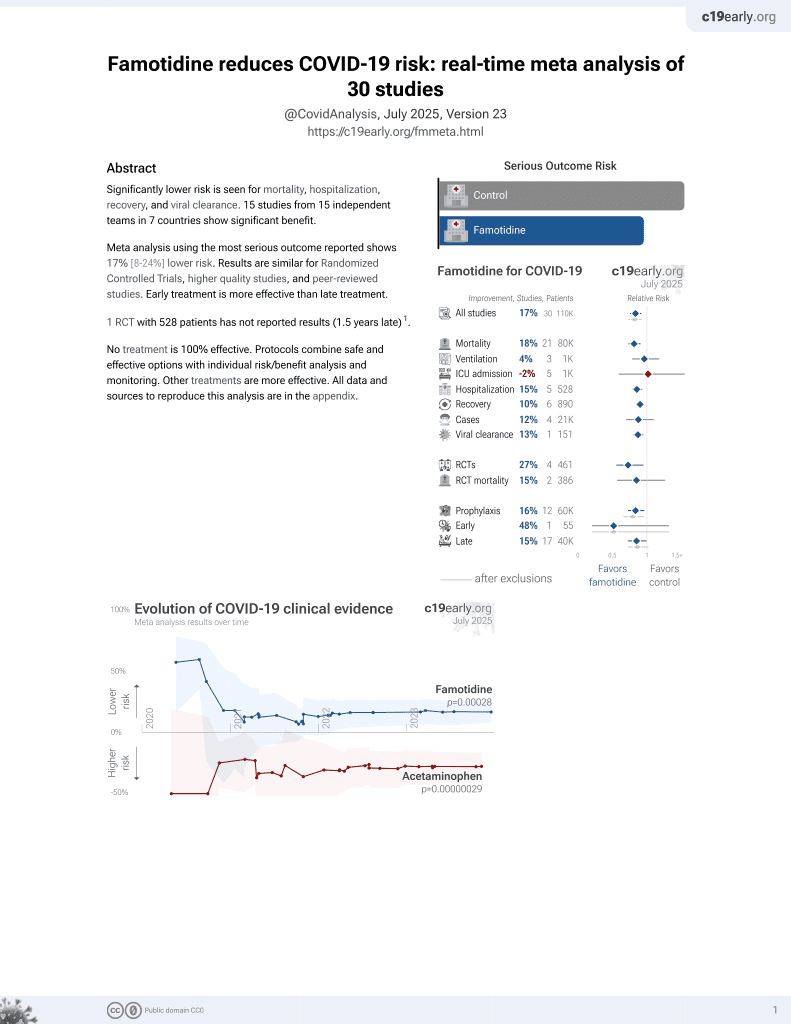
Famotidine for COVID-19
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Survey of 307 patients in the USA, showing no significant difference in COVID-19 cases with famotidine use.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of symptomatic case, 22.0% lower, RR 0.78, p = 0.49, treatment 18 of 80 (22.5%), control 49 of 227 (21.6%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
recovery time, 36.9% lower, relative time 0.63, p = 0.32, treatment 80, control 227.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Balouch et al., 20 Jan 2021, retrospective, USA, peer-reviewed, 5 authors.
Role of Famotidine and Other Acid Reflux Medications for SARS-CoV-2: A Pilot Study
Journal of Voice, doi:10.1016/j.jvoice.2021.01.007
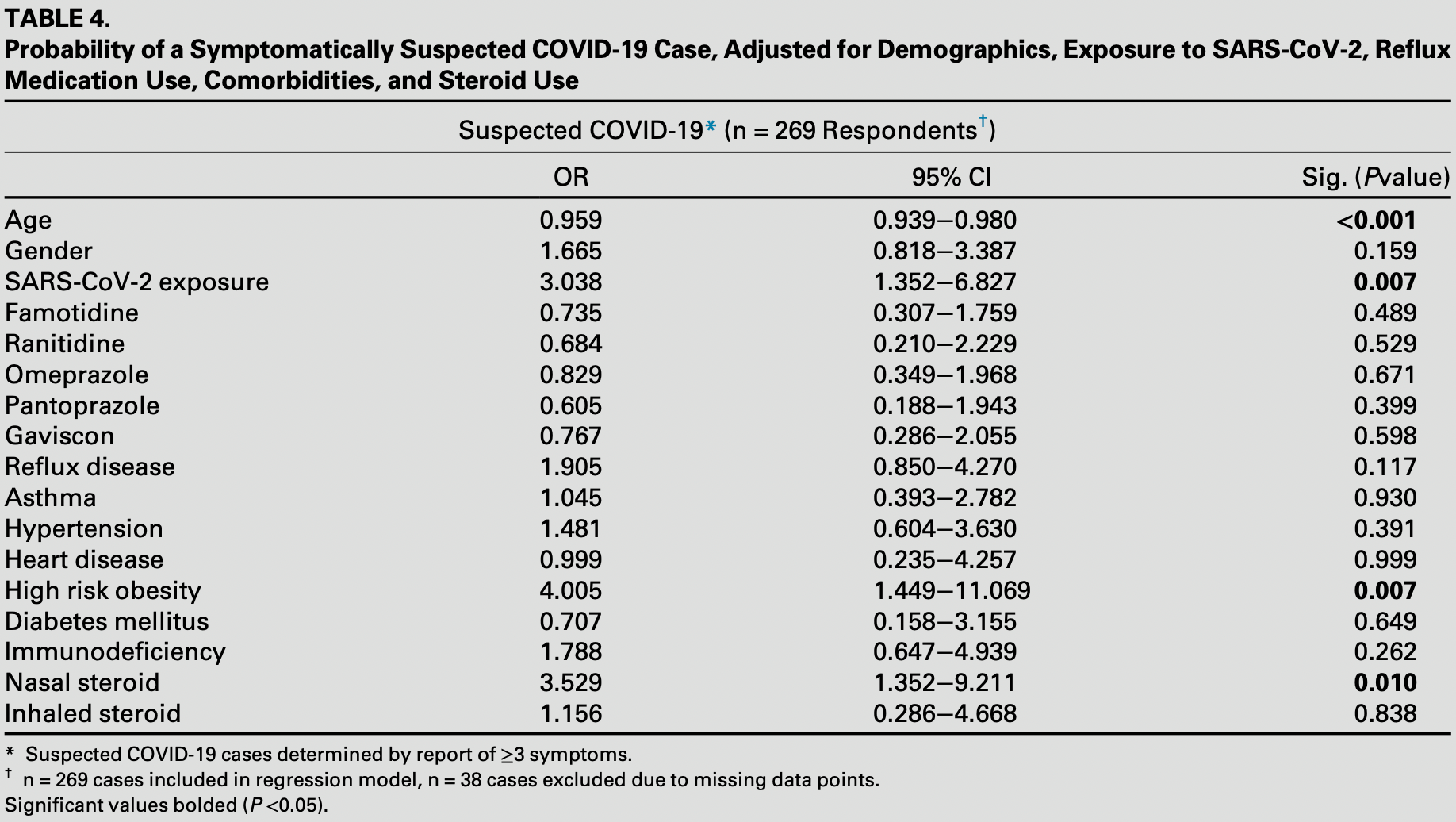
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus-19 disease (COVID-19) pandemic. The H-2 blocker famotidine has been suggested as an FDAapproved drug that could potentially be repurposed for treatment of COVID-19. Famotidine has since been shown to improve patient outcomes and reduce symptom severity in patients acutely ill with COVID-19. Other studies have suggested that proton pump inhibitors (PPIs) might have an association with COVID-19. Objective. The purpose of the present study was to determine whether famotidine or any other antireflux medications have a prophylactic or detrimental effect for SARS-CoV-2 infection when taken regularly for the management of acid reflux. Methods. An anonymous, web-based survey was distributed via email to adult otolaryngology patients to collect demographic data, past medical history, medication history, incidence of symptoms associated with COVID-19, potential exposure to SARS-CoV-2, and results of any PCR or serological testing. Associations between reflux medications and incidence of COVID-19 cases were analyzed. Statistical analysis was performed using SPSS. Chisquare with Fisher's exact test, Point-Biserial correlation, Kendall's-tau-b, independent samples t test, and the Mann-Whitney U test were used as appropriate. A binary logistic regression model was fit to determine probability of COVID-19 cases after adjustment for other risk factors. Results. There were 307 patients who responded to the survey. The average age of respondents was 52.63 § 17.03. Famotidine use was not associated with incidence of laboratory-confirmed (P= 0.717) or symptomatically suspected (P= 0.876) COVID-19. No other reflux medications were found to be significant predictors for laboratory-confirmed or suspected COVID-19 (P> 0.05). Younger age (odds ratio [OR] = 1.043, 95% CI: 1.020−1.065, P< 0.001), high risk obesity (OR = 4.005, 95% CI: 1.449−11.069, P= 0.007), and use of a corticosteroid nasal spray (OR = 3.529, 95% CI: 1.352−9.211, P= 0.010) were significant predictors for symptomatically suspected COVID-19 cases. Conclusions. There was no association between incidence of COVID-19 and use of reflux medications, including famotidine at doses used orally to manage reflux and high dose PPIs. Reflux medications did not protect against or increase the risk of COVID-19.
References
Boehmer, Devies, Caruso, Changing age distribution of the COVID-19 pandemic -United States, MMWR Morb Mortal Wkly Rep
Bojkova, Mcgreig, Mclaughlin, SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles
Bray, Pathophysiology of obesity, Am J Clin Nutr
Farrell, Klatt-Cromwell, Schneider, Benefits and safety of nasal saline irrigations in a pandemic-washing COVID-19 away, JAMA Otolaryngol Head Neck Surg
Freedberg, Conigliaro, Wang, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology
Fresan, Guevara, Elia, Independent role of morbid obesity as a risk factor for COVID-19 hospitalization: a Spanish population-based cohort study, Obesity (Silver Spring)
Friedenberg, Xanthopoulos, Foster, The association between gastroesophageal reflux disease and obesity, Am J Gastroenterol
Gupta, Biswal, Singha, Binding insight of clinically oriented drug famotidine with the identified potential target of SARS-CoV-2, J Biomol Struct Dyn
Gupta, Hayek, Wang, Factors associated with death in critically ill patients with coronavirus disease 2019 in the US, JAMA Intern Med
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inform
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)−a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Herman, Vincent, Winkler, Consensus statement. Corticosteroid therapy in ENT in the context of the COVID-19 pandemic, Eur Ann Otorhinolaryngol Head Neck Dis
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut
Karlsson, Beck, The burden of obesity on infectious disease, Exp Biol Med (Maywood)
Lan, Filler, Mathew, COVID-19 symptoms predictive of healthcare workers' SARS-CoV-2 PCR results, PLoS One
Lechien, Huet, Khalife, Alkaline, protein, low-fat and low-acid diet in laryngopharyngeal reflux disease: our experience on 65 patients, Clin Otolaryngol
Lee, Ha, Yeniova, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut
Luxenburger, Sturm, Biever, Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor?, J Intern Med
Mather, Seip, Mckay, Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19, Am J Gastroenterol
Ortega, Serrano, Jastrzebska, Class A g protein-coupled receptor antagonist famotidine as a therapeutic alternative against SARS-CoV2: an in silico analysis, Biomolecules
Popkin, Du, Green, Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships, Obes Rev
Sandhu, Fass, Current trends in the management of gastroesophageal reflux disease, Gut Liver
Sataloff, Castell, Katz, Reflux and other gastroenterologic conditions that may affect the voice. Obesity and the Voice
Who, World Health Organization Web site
Wu, Liu, Yang, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B
DOI record:
{
"DOI": "10.1016/j.jvoice.2021.01.007",
"ISSN": [
"0892-1997"
],
"URL": "http://dx.doi.org/10.1016/j.jvoice.2021.01.007",
"alternative-id": [
"S0892199721000321"
],
"author": [
{
"affiliation": [],
"family": "Balouch",
"given": "Bailey",
"sequence": "first"
},
{
"affiliation": [],
"family": "Vontela",
"given": "Swetha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yeakel",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alnouri",
"given": "Ghiath",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sataloff",
"given": "Robert T.",
"sequence": "additional"
}
],
"container-title": [
"Journal of Voice"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
1,
20
]
],
"date-time": "2021-01-20T14:01:15Z",
"timestamp": 1611151275000
},
"deposited": {
"date-parts": [
[
2021,
1,
28
]
],
"date-time": "2021-01-28T20:39:01Z",
"timestamp": 1611866341000
},
"indexed": {
"date-parts": [
[
2021,
12,
8
]
],
"date-time": "2021-12-08T22:58:37Z",
"timestamp": 1639004317374
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0892-1997"
}
],
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0892199721000321?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0892199721000321?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jvoice.2021.01.007_bib0001",
"series-title": "Coronavirus Disease (COVID-19). World Health Organization Web site",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"article-title": "Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study",
"author": "Freedberg",
"doi-asserted-by": "crossref",
"first-page": "1129",
"journal-title": "Gastroenterology",
"key": "10.1016/j.jvoice.2021.01.007_bib0002",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.14309/ajg.0000000000000832",
"article-title": "Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19",
"author": "Mather",
"doi-asserted-by": "crossref",
"first-page": "1617",
"journal-title": "Am J Gastroenterol",
"key": "10.1016/j.jvoice.2021.01.007_bib0003",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"article-title": "Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series",
"author": "Janowitz",
"doi-asserted-by": "crossref",
"first-page": "1592",
"journal-title": "Gut",
"key": "10.1016/j.jvoice.2021.01.007_bib0004",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-322248",
"article-title": "Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Gut",
"key": "10.1016/j.jvoice.2021.01.007_bib0005",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1111/joim.13121",
"article-title": "Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor?",
"author": "Luxenburger",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "J Intern Med",
"key": "10.1016/j.jvoice.2021.01.007_bib0006",
"volume": "289",
"year": "2020"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.jvoice.2021.01.007_bib0007",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners",
"author": "Harris",
"doi-asserted-by": "crossref",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.jvoice.2021.01.007_bib0008",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0235460",
"article-title": "COVID-19 symptoms predictive of healthcare workers' SARS-CoV-2 PCR results",
"author": "Lan",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.jvoice.2021.01.007_bib0009",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1093/ajcn/55.2.488s",
"article-title": "Pathophysiology of obesity",
"author": "Bray",
"doi-asserted-by": "crossref",
"first-page": "488S",
"issue": "2 Suppl",
"journal-title": "Am J Clin Nutr",
"key": "10.1016/j.jvoice.2021.01.007_bib0010",
"volume": "55",
"year": "1992"
},
{
"DOI": "10.1111/coa.13269",
"article-title": "Alkaline, protein, low-fat and low-acid diet in laryngopharyngeal reflux disease: our experience on 65 patients",
"author": "Lechien",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Clin Otolaryngol",
"key": "10.1016/j.jvoice.2021.01.007_bib0011",
"volume": "44",
"year": "2019"
},
{
"DOI": "10.5009/gnl16615",
"article-title": "Current trends in the management of gastroesophageal reflux disease",
"author": "Sandhu",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Gut Liver",
"key": "10.1016/j.jvoice.2021.01.007_bib0012",
"volume": "12",
"year": "2018"
},
{
"article-title": "Reflux and other gastroenterologic conditions that may affect the voice",
"author": "Sataloff",
"first-page": "183",
"key": "10.1016/j.jvoice.2021.01.007_bib0013",
"series-title": "Obesity and the Voice",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"article-title": "Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "Acta Pharm Sin B",
"key": "10.1016/j.jvoice.2021.01.007_bib0014",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3390/biom10060954",
"article-title": "Class A g protein-coupled receptor antagonist famotidine as a therapeutic alternative against SARS-CoV2: an in silico analysis",
"author": "Ortega",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Biomolecules",
"key": "10.1016/j.jvoice.2021.01.007_bib0015",
"volume": "10",
"year": "2020"
},
{
"article-title": "Binding insight of clinically oriented drug famotidine with the identified potential target of SARS-CoV-2",
"author": "Sen Gupta",
"first-page": "1",
"journal-title": "J Biomol Struct Dyn",
"key": "10.1016/j.jvoice.2021.01.007_bib0016",
"year": "2020"
},
{
"author": "Bojkova",
"key": "10.1016/j.jvoice.2021.01.007_bib0017",
"series-title": "SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6939e1",
"article-title": "Changing age distribution of the COVID-19 pandemic — United States",
"author": "Boehmer",
"doi-asserted-by": "crossref",
"first-page": "1404",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.jvoice.2021.01.007_bib0018",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.3596",
"article-title": "Factors associated with death in critically ill patients with coronavirus disease 2019 in the US",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.jvoice.2021.01.007_bib0019",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1111/obr.13128",
"article-title": "Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships",
"author": "Popkin",
"doi-asserted-by": "crossref",
"first-page": "e13128",
"journal-title": "Obes Rev",
"key": "10.1016/j.jvoice.2021.01.007_bib0020",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1002/oby.23029",
"article-title": "Independent role of morbid obesity as a risk factor for COVID-19 hospitalization: a Spanish population-based cohort study",
"author": "Fresan",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Obesity (Silver Spring)",
"key": "10.1016/j.jvoice.2021.01.007_bib0021",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1258/ebm.2010.010227",
"article-title": "The burden of obesity on infectious disease",
"author": "Karlsson",
"doi-asserted-by": "crossref",
"first-page": "1412",
"journal-title": "Exp Biol Med (Maywood)",
"key": "10.1016/j.jvoice.2021.01.007_bib0022",
"volume": "235",
"year": "2010"
},
{
"DOI": "10.1111/j.1572-0241.2008.01946.x",
"article-title": "The association between gastroesophageal reflux disease and obesity",
"author": "Friedenberg",
"doi-asserted-by": "crossref",
"first-page": "2111",
"journal-title": "Am J Gastroenterol",
"key": "10.1016/j.jvoice.2021.01.007_bib0023",
"volume": "103",
"year": "2008"
},
{
"DOI": "10.1016/j.anorl.2020.04.014",
"article-title": "Consensus statement. Corticosteroid therapy in ENT in the context of the COVID-19 pandemic",
"author": "Herman",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Eur Ann Otorhinolaryngol Head Neck Dis",
"key": "10.1016/j.jvoice.2021.01.007_bib0024",
"volume": "137",
"year": "2020"
},
{
"DOI": "10.1001/jamaoto.2020.1622",
"article-title": "Benefits and safety of nasal saline irrigations in a pandemic-washing COVID-19 away",
"author": "Farrell",
"doi-asserted-by": "crossref",
"first-page": "787",
"journal-title": "JAMA Otolaryngol Head Neck Surg",
"key": "10.1016/j.jvoice.2021.01.007_bib0025",
"volume": "146",
"year": "2020"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"score": 1,
"short-container-title": [
"Journal of Voice"
],
"short-title": [],
"source": "Crossref",
"subject": [
"LPN and LVN",
"Speech and Hearing",
"Otorhinolaryngology"
],
"subtitle": [],
"title": [
"Role of Famotidine and Other Acid Reflux Medications for SARS-CoV-2: A Pilot Study"
],
"type": "journal-article"
}