Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial
et al., Gut, doi:10.1136/gutjnl-2022-326952, NCT04724720, Feb 2022
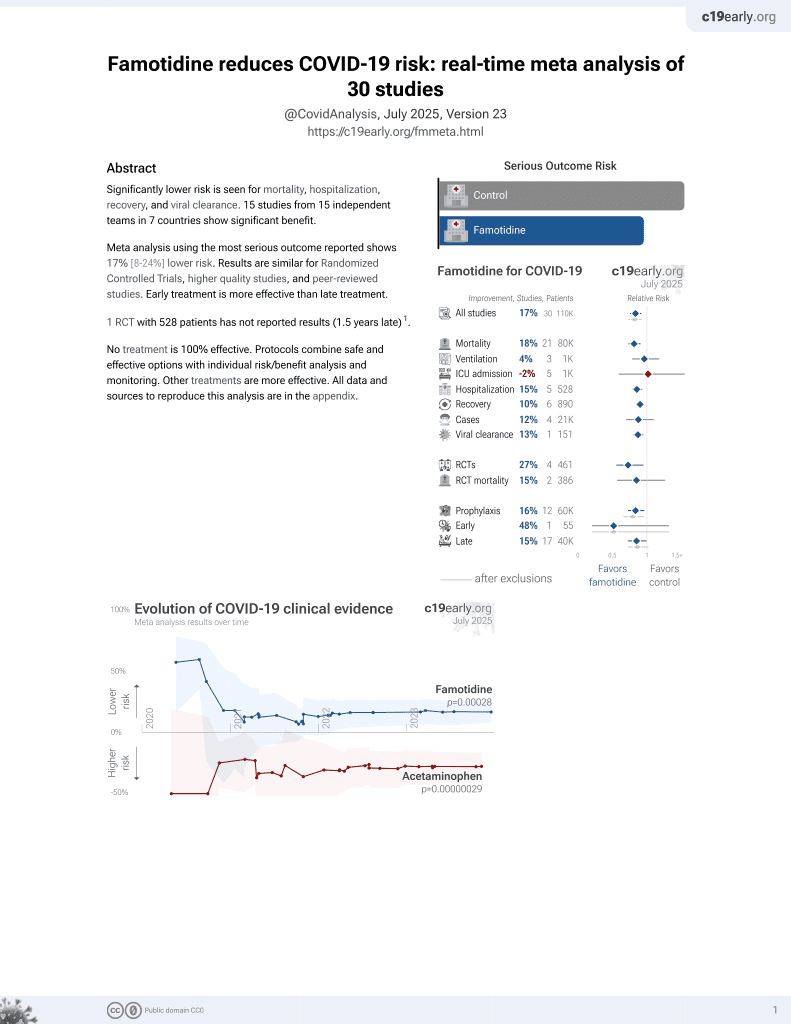
Famotidine for COVID-19
28th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
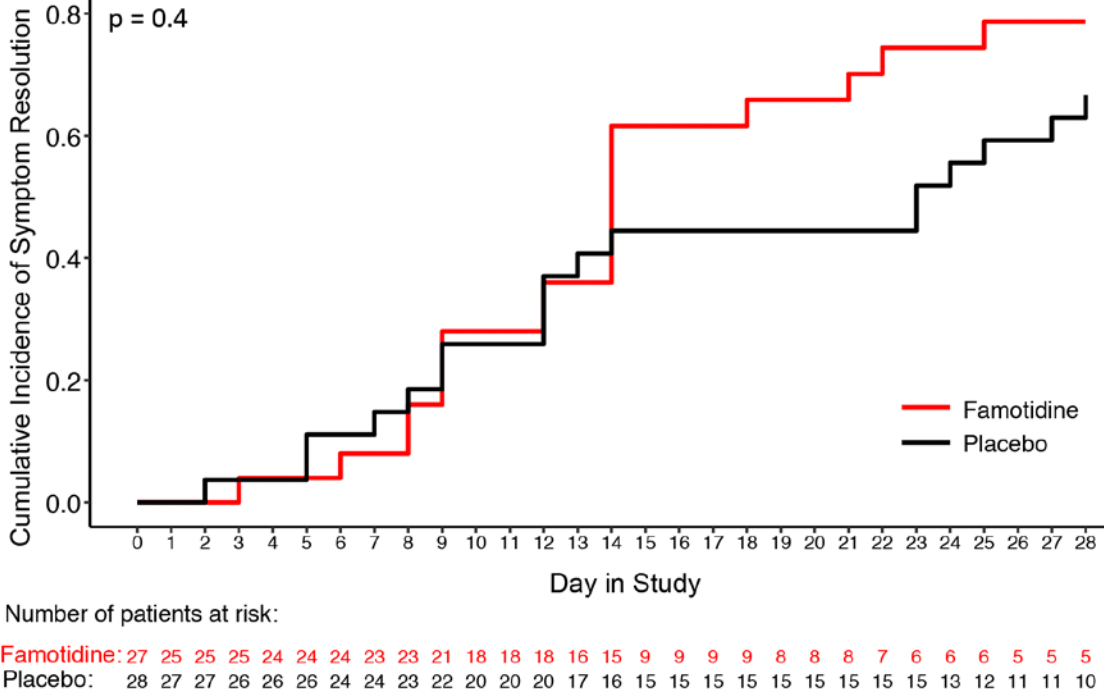
Small RCT with 27 famotidine and 28 placebo patients, showing improved recovery with treatment. Recovery was faster with treatment for 14 of 16 symptoms. There was no mortality or hospitalization. NCT04724720 (history).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no recovery, 48.1% lower, RR 0.52, p = 0.23, treatment 5 of 27 (18.5%), control 10 of 28 (35.7%), NNT 5.8, day 28, ITT.
|
|
risk of no recovery, 43.2% lower, RR 0.57, p = 0.34, treatment 4 of 19 (21.1%), control 10 of 27 (37.0%), NNT 6.3, day 28, PP.
|
|
estimated time to 50% resolution, 28.1% lower, relative time 0.72, p < 0.01, treatment 27, control 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Brennan et al., 10 Feb 2022, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 31 authors, study period January 2021 - April 2021, average treatment delay 4.0 days, trial NCT04724720 (history).
Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial
Gut, doi:10.1136/gutjnl-2022-326952
Objective We assessed whether famotidine improved inflammation and symptomatic recovery in outpatients with mild to moderate COVID-19. Design Randomised, double-blind, placebocontrolled, fully remote, phase 2 clinical trial (NCT04724720) enrolling symptomatic unvaccinated adult outpatients with confirmed COVID-19 between January 2021 and April 2021 from two US centres. Patients self-administered 80 mg famotidine (n=28) or placebo (n=27) orally three times a day for 14 consecutive days. Endpoints were time to (primary) or rate of (secondary) symptom resolution, and resolution of inflammation (exploratory). Results Of 55 patients in the intention-to-treat group (median age 35 years (IQR: 20); 35 women (64%); 18 African American (33%); 14 Hispanic (26%)), 52 (95%) completed the trial, submitting 1358 electronic symptom surveys. Time to symptom resolution was not statistically improved (p=0.4). Rate of symptom resolution was improved for patients taking famotidine (p<0.0001). Estimated 50% reduction of overall baseline symptom scores were achieved at 8.2 days (95% CI: 7 to 9.8 days) for famotidine and 11.4 days (95% CI: 10.3 to 12.6 days) for placebo treated patients. Differences were independent of patient sex, race or ethnicity. Five self-limiting adverse events occurred (famotidine, n=2 (40%); placebo, n=3 (60%)). On day 7, fewer patients on famotidine had detectable interferon alpha plasma levels (p=0.04). Plasma immunoglobulin type G levels to SARS-CoV-2 nucleocapsid core protein were similar between both arms. Conclusions Famotidine was safe and well tolerated in outpatients with mild to moderate COVID-19. Famotidine led to earlier resolution of symptoms and inflammation without reducing anti-SARS-CoV-2 immunity. Additional randomised trials are required.
INTRODUCTION The search for safe, effective and affordable treatments for COVID-19 remains a global health priority. COVID-19 is pandemic, with an estimated 319 M cases and 5.5 M deaths worldwide to date. Restrictive public health measures in response to COVID-19 have led to unprecedented negative impacts on society. 2 COVID-19 is caused by the SARS-CoV-2. On body entry, SARS-CoV-2 docks to the widely expressed ACE2 4 5 and is internalised into the cell. Viral replication causes cell death and engages the immune system. 8 Toll-like receptor 3 (TLR3) binds
Significance of this study What is already known on this subject? ► COVID-19 is caused by the SARS-CoV-2. ► Cytokine release drives inflammation and poor outcome in patients with COVID-19. ► Famotidine is a histamine 2 receptor antagonist that is globally used to reduce gastric reflux symptoms and treat gastric ulcers. ► In laboratory studies, famotidine reduced type-I interferon release from virally infected epithelial cells. ► Famotidine improved the outcome of patients with COVID-19 in some retrospective studies and a case series, but evidence from a clinical trial is lacking.
What are the new findings? ► In this randomised,..
Intestinal inflammation the experimental medicine part of the study. DAT and TJ secured funding. All authors read, edited and approved the final manuscript. Competing interests None declared.
Patient consent for publication Not applicable. Ethics approval This study involves human participants and was approved by The Northwell Health Institutional Review Board IRB#: 20-1155. Participants gave informed consent to participate in the study before taking part. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available upon reasonable request. The gene list for assessing type-I interferon response is provided in Table S5 . Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Open access This is an open access article distributed in accordance with the Creative Commons..
References
Bastard, Rosen, Zhang, Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science, doi:10.1126/science.abd4585
Bayati, Kumar, Francis, SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis, J Biol Chem, doi:10.1016/j.jbc.2021.100306
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bland, Altman, The logrank test, BMJ, doi:10.1136/bmj.328.7447.1073
Bouwhuis, Collette, Suciu, Changes of ferritin and CRP levels in melanoma patients treated with adjuvant interferon-α (EORTC 18952) and prognostic value on treatment outcome, Melanoma Res, doi:10.1097/CMR.0b013e328346c17f
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Channappanavar, Fehr, Vijay, Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoVinfected mice, Cell Host Microbe, doi:10.1016/j.chom.2016.01.007
Channappanavar, Fehr, Zheng, Ifn-I response timing relative to virus replication determines MERS coronavirus infection outcomes, J Clin Invest, doi:10.1172/JCI126363
Cohn, Cirillo, Murphy, SARS-CoV-2 vaccine protection and deaths among US veterans during 2021, Science, doi:10.1126/science.abm0620
Collie, Champion, Moultrie, Effectiveness of BNT162b2 vaccine against omicron variant in South Africa, N Engl J Med, doi:10.1056/NEJMc2119270
Diggle, Diggle, Analysis of longitudinal data
Dobin, Davis, Schlesinger, Star: ultrafast universal RNA-seq aligner, Bioinformatics, doi:10.1093/bioinformatics/bts635
Douglas, Katikireddi, Taulbut, Mitigating the wider health effects of covid-19 pandemic response, BMJ, doi:10.1136/bmj.m1557
Ewels, Peltzer, Fillinger, The nf-core framework for community-curated bioinformatics pipelines, Nat Biotechnol, doi:10.1038/s41587-020-0439-x
Eyre, Taylor, Purver, Effect of Covid-19 vaccination on transmission of alpha and delta variants, N Engl J Med, doi:10.1056/NEJMoa2116597
Fajgenbaum, June, Cytokine storm, N Engl J Med, doi:10.1056/NEJMra2026131
Freedberg, Conigliaro, Wang, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Furman, Campisi, Verdin, Chronic inflammation in the etiology of disease across the life span, Nat Med, doi:10.1038/s41591-019-0675-0
Gottlieb, Vaca, Paredes, Early Remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Hadjadj, Yatim, Barnabei, Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science, doi:10.1038/s41586-021-03234-7
Henry, De Oliveira, Benoit, Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis, Clin Chem Lab Med, doi:10.1515/cclm-2020-0369
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Hänzelmann, Castelo, Guinney, GSVA: gene set variation analysis for microarray and RNA-Seq data, BMC Bioinformatics, doi:10.1186/1471-2105-14-7
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut, doi:10.1136/gutjnl-2020-321852
Karki, Sharma, Tuladhar, Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes, Cell, doi:10.1016/j.cell.2020.11.025
Lan, Ge, Yu, Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Li, Dewey, RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome, BMC Bioinformatics, doi:10.1186/1471-2105-12-323
Loffredo, Lucero, Chen, The in-vitro effect of famotidine on sars-cov-2 proteases and virus replication, Sci Rep, doi:10.1038/s41598-021-84782-w
Mather, Seip, Mckay, Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19, Am J Gastroenterol, doi:10.14309/ajg.0000000000000832
Miwa, Tani, Miwa, Brennan, Cm, Inhibition of gastric secretion by a new H2-antagonist, YM-11170 in healthy subjects, Int J Clin Pharmacol Ther Toxicol, doi:10.1016/j.it.2021.02.003
Mukherjee, Bhattacharya, Bojkova, Famotidine inhibits Toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection, J Biol Chem, doi:10.1016/j.jbc.2021.100925
Osterberg, Blaschke, Adherence to medication, N Engl J Med, doi:10.1056/NEJMra050100
Park, Iwasaki, Type I and Type III Interferons -Induction, Signaling, Evasion, and Application to Combat COVID-19, Cell Host Microbe, doi:10.1016/j.chom.2020.05.008
Pritchard, Matthews, Stoesser, Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom, Nat Med, doi:10.1038/s41591-021-01410-w
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the together randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/S2214-109X(21)00448-4
Shang, Ye, Shi, Structural basis of receptor recognition by SARS-CoV-2, Nature, doi:10.1038/s41586-020-2179-y
Shelton, Shastri, Ye, Trans-Ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity, Nat Genet, doi:10.1038/s41588-021-00854-7
Sinha, Rosin, Arora, Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19, Nat Med, doi:10.1038/s41591-021-01576-3
Smid, Van Den Braak, Van De Werken, Gene length corrected trimmed mean of m-values (GeTMM) processing of RNA-Seq data performs similarly in intersample analyses while improving intrasample comparisons, BMC Bioinformatics, doi:10.1186/s12859-018-2246-7
Stam, Swaak, Kruit, Regulation of ferritin: a specific role for interferon-alpha (IFN-alpha)? the acute phase response in patients treated with IFNalpha-2b, Eur J Clin Invest, doi:10.1046/j.1365-2362.2002.0320s1079.x
Tay, Poh, Rénia, The trinity of COVID-19: immunity, inflammation and intervention, Nat Rev Immunol, doi:10.1038/s41577-020-0311-8
Virgin, Wherry, Ahmed, Redefining chronic viral infection, Cell, doi:10.1016/j.cell.2009.06.036
Wang, Zhan, Zhu, Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients, Cell Host Microbe, doi:10.1016/j.chom.2020.07.005
Wouters, Shadlen, Salcher-Konrad, Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment, Lancet, doi:10.1016/S0140-6736(21)00306-8
Yeramaneni, Doshi, Sands, Famotidine use is not associated with 30-day mortality: a Coarsened exact match study in 7158 hospitalized patients with coronavirus disease 2019 from a large healthcare system, Gastroenterology, doi:10.1053/j.gastro.2020.10.011
Zhang, Bastard, Liu, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science, doi:10.1126/science.abd4570
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
DOI record:
{
"DOI": "10.1136/gutjnl-2022-326952",
"ISSN": [
"0017-5749",
"1468-3288"
],
"URL": "http://dx.doi.org/10.1136/gutjnl-2022-326952",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>We assessed whether famotidine improved inflammation and symptomatic recovery in outpatients with mild to moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Randomised, double-blind, placebo-controlled, fully remote, phase 2 clinical trial (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04724720\">NCT04724720</jats:ext-link>) enrolling symptomatic unvaccinated adult outpatients with confirmed COVID-19 between January 2021 and April 2021 from two US centres. Patients self-administered 80 mg famotidine (n=28) or placebo (n=27) orally three times a day for 14 consecutive days. Endpoints were time to (primary) or rate of (secondary) symptom resolution, and resolution of inflammation (exploratory).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of 55 patients in the intention-to-treat group (median age 35 years (IQR: 20); 35 women (64%); 18 African American (33%); 14 Hispanic (26%)), 52 (95%) completed the trial, submitting 1358 electronic symptom surveys. Time to symptom resolution was not statistically improved (p=0.4). Rate of symptom resolution was improved for patients taking famotidine (p<0.0001). Estimated 50% reduction of overall baseline symptom scores were achieved at 8.2 days (95% CI: 7 to 9.8 days) for famotidine and 11.4 days (95% CI: 10.3 to 12.6 days) for placebo treated patients. Differences were independent of patient sex, race or ethnicity. Five self-limiting adverse events occurred (famotidine, n=2 (40%); placebo, n=3 (60%)). On day 7, fewer patients on famotidine had detectable interferon alpha plasma levels (p=0.04). Plasma immunoglobulin type G levels to SARS-CoV-2 nucleocapsid core protein were similar between both arms.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Famotidine was safe and well tolerated in outpatients with mild to moderate COVID-19. Famotidine led to earlier resolution of symptoms and inflammation without reducing anti-SARS-CoV-2 immunity. Additional randomised trials are required.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/gutjnl-2022-326952"
],
"author": [
{
"affiliation": [],
"family": "Brennan",
"given": "Christina M",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nadella",
"given": "Sandeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Xiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dima",
"given": "Richard J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jordan-Martin",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demestichas",
"given": "Breanna R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kleeman",
"given": "Sam O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "von Gablenz",
"given": "Eva Carlotta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourikis",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rubin",
"given": "Michael E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adnani",
"given": "Harsha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Hassal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4533-1140",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ha",
"given": "Taehoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prum",
"given": "Soma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schleicher",
"given": "Cheryl B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Sharon S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ryan",
"given": "Michael G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pili",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldberg",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crawford",
"given": "James M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodwin",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Xiaoyue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Preall",
"given": "Jonathan B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costa",
"given": "Ana S H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conigliaro",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masci",
"given": "Joseph R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Jie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8017-2712",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tuveson",
"given": "David A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tracey",
"given": "Kevin J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7820-3727",
"affiliation": [],
"authenticated-orcid": false,
"family": "Janowitz",
"given": "Tobias",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04724720",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04724720",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": [
"Gut"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T18:20:37Z",
"timestamp": 1644517237000
},
"deposited": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T18:20:57Z",
"timestamp": 1644517257000
},
"funder": [
{
"award": [
"100010434"
],
"name": "La Caixa Foundation Fellowship"
},
{
"award": [
"R35GM118182-06"
],
"name": "NIH"
},
{
"award": [
"N/A"
],
"name": "MRC CU Research Studentship"
},
{
"award": [
"N/A"
],
"name": "Starr Centennial Scholarship at CSHL"
},
{
"award": [
"5P30CA045508"
],
"name": "Cancer Center Support Grant"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T18:42:21Z",
"timestamp": 1644518541425
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0017-5749"
},
{
"type": "electronic",
"value": "1468-3288"
}
],
"issued": {
"date-parts": [
[
2022,
2,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T00:00:00Z",
"timestamp": 1644364800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/gutjnl-2022-326952",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "gutjnl-2022-326952",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
2,
10
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
10
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2022021010201279000_gutjnl-2022-326952v1.1",
"unstructured": "World Health Organization . Who COVID-19 explorer. Geneva, 2020. https://worldhealthorg.shinyapps.io/covid/"
},
{
"DOI": "10.1136/bmj.m1557",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.2"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.3"
},
{
"DOI": "10.1038/s41586-020-2179-y",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.4"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.5"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.6"
},
{
"DOI": "10.1016/j.jbc.2021.100306",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.7"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.8"
},
{
"DOI": "10.1016/j.jbc.2021.100925",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.9"
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.10"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.11"
},
{
"DOI": "10.1038/s41591-019-0675-0",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.12"
},
{
"DOI": "10.1038/s41588-021-00854-7",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.13"
},
{
"DOI": "10.1126/science.abm0620",
"article-title": "SARS-CoV-2 vaccine protection and deaths among US veterans during 2021",
"author": "Cohn",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "2022021010201279000_gutjnl-2022-326952v1.14",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01410-w",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.15"
},
{
"article-title": "Effectiveness of BNT162b2 vaccine against omicron variant in South Africa",
"author": "Collie",
"journal-title": "N Engl J Med",
"key": "2022021010201279000_gutjnl-2022-326952v1.16",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116597",
"article-title": "Effect of Covid-19 vaccination on transmission of alpha and delta variants",
"author": "Eyre",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "2022021010201279000_gutjnl-2022-326952v1.17",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00306-8",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.18"
},
{
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"journal-title": "N Engl J Med",
"key": "2022021010201279000_gutjnl-2022-326952v1.19",
"year": "2021"
},
{
"key": "2022021010201279000_gutjnl-2022-326952v1.20",
"unstructured": "FDA . Fact sheet for healthcare providers: emergency use authorization for Paxlovid, 2021. Available: https://www.fda.gov/media/155050/download [Accessed 17 Jan 2022]."
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.21"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.22"
},
{
"DOI": "10.1038/s41591-021-01576-3",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.23"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.24"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.25"
},
{
"DOI": "10.14309/ajg.0000000000000832",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.26"
},
{
"DOI": "10.1053/j.gastro.2020.10.011",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.27"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.28"
},
{
"DOI": "10.1136/bmj.328.7447.1073",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.29"
},
{
"DOI": "10.1038/s41587-020-0439-x",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.30"
},
{
"DOI": "10.1093/bioinformatics/bts635",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.31"
},
{
"DOI": "10.1186/1471-2105-12-323",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.32"
},
{
"DOI": "10.1186/s12859-018-2246-7",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.33"
},
{
"DOI": "10.1186/1471-2105-14-7",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.34"
},
{
"key": "2022021010201279000_gutjnl-2022-326952v1.35",
"unstructured": "FDA . Food and drug administration Accessdata famotidine information reference ID: 2954009, 2011."
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "2022021010201279000_gutjnl-2022-326952v1.36",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-84782-w",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.37"
},
{
"key": "2022021010201279000_gutjnl-2022-326952v1.38",
"unstructured": "Diggle P , Diggle P . Analysis of longitudinal data. 2nd edn. Oxford University Press, 2002."
},
{
"DOI": "10.1016/j.cell.2009.06.036",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.39"
},
{
"DOI": "10.1046/j.1365-2362.2002.0320s1079.x",
"article-title": "Regulation of ferritin: a specific role for interferon-alpha (IFN-alpha)? the acute phase response in patients treated with IFN-alpha-2b",
"author": "Stam",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Eur J Clin Invest",
"key": "2022021010201279000_gutjnl-2022-326952v1.40",
"volume": "32 Suppl 1",
"year": "2002"
},
{
"DOI": "10.1097/CMR.0b013e328346c17f",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.41"
},
{
"DOI": "10.1515/cclm-2020-0369",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.42"
},
{
"DOI": "10.1056/NEJMra050100",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.43"
},
{
"article-title": "Inhibition of gastric secretion by a new H2-antagonist, YM-11170 in healthy subjects",
"author": "Miwa",
"first-page": "214",
"journal-title": "Int J Clin Pharmacol Ther Toxicol",
"key": "2022021010201279000_gutjnl-2022-326952v1.44",
"volume": "22",
"year": "1984"
},
{
"DOI": "10.1016/j.it.2021.02.003",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.45"
},
{
"DOI": "10.1126/science.abc6027",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.46"
},
{
"DOI": "10.1038/s41586-021-03234-7",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.47"
},
{
"DOI": "10.1126/science.abd4585",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.48"
},
{
"DOI": "10.1126/science.abd4570",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.49"
},
{
"DOI": "10.1016/j.chom.2020.05.008",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.50"
},
{
"DOI": "10.1016/j.chom.2016.01.007",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.51"
},
{
"DOI": "10.1172/jci126363",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.52"
},
{
"DOI": "10.1016/j.chom.2020.07.005",
"doi-asserted-by": "publisher",
"key": "2022021010201279000_gutjnl-2022-326952v1.53"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"score": 1,
"short-container-title": [
"Gut"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology"
],
"subtitle": [],
"title": [
"Oral famotidine versus placebo in non-hospitalised patients with COVID-19: a randomised, double-blind, data-intense, phase 2 clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}