Atovaquone for treatment of COVID-19: A prospective randomized, double-blind, placebo-controlled clinical trial
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.1020123, NCT04456153, Sep 2022
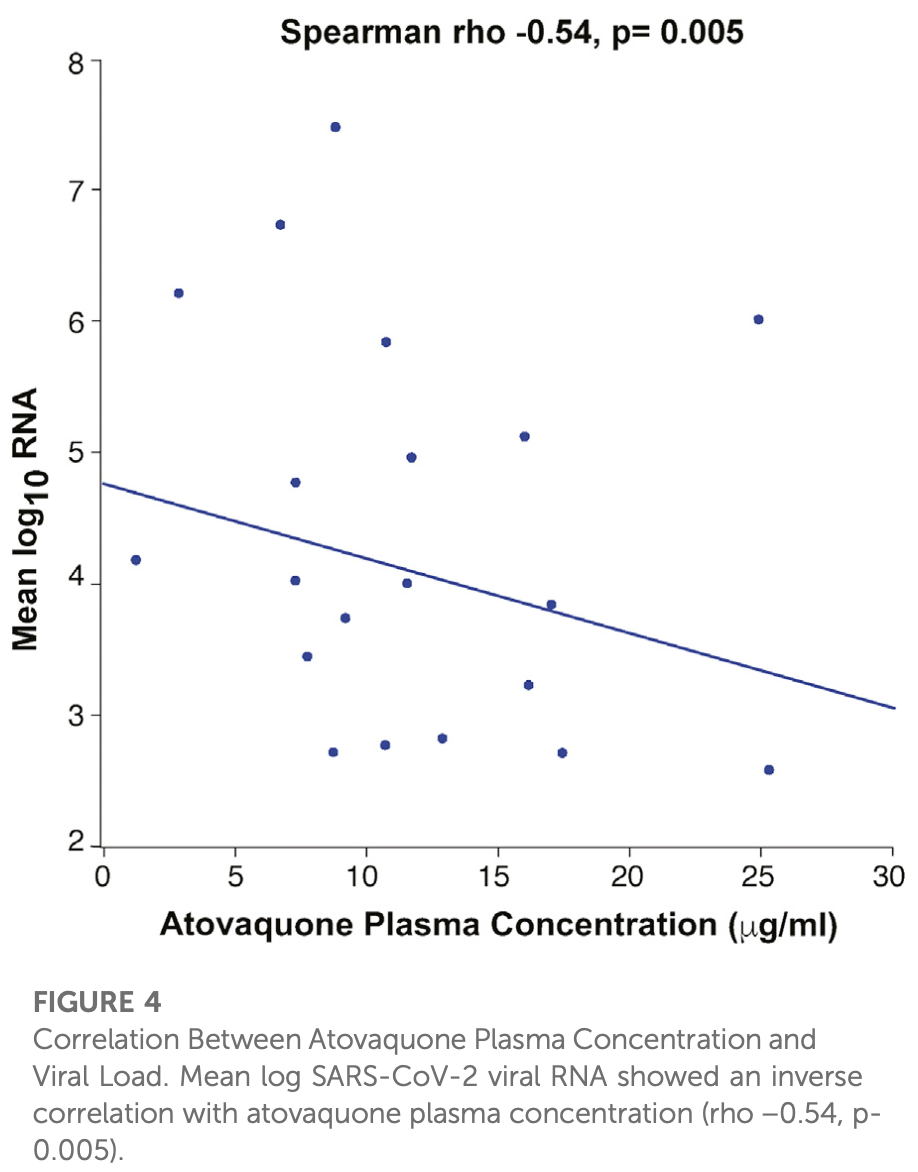
RCT 60 hospitalized COVID-19 patients showing no significant difference in viral clearance with atovaquone. Patients were randomized 2:1 to receive atovaquone 1500mg BID or placebo for up to 10 days, with both groups receiving standard of care treatments including remdesivir and dexamethasone. Pharmacokinetic data revealed that adequate inhibitory drug concentrations were likely not achieved in most patients by day 3, with higher BMI negatively correlating with drug levels. Authors observed an inverse correlation between atovaquone plasma concentration and viral load, suggesting a potential antiviral effect might be possible with adequate dosing.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 39.0% higher, RR 1.39, p = 1.00, treatment 6 of 41 (14.6%), control 2 of 19 (10.5%).
|
|
risk of no improvement, 20.3% lower, RR 0.80, p = 0.56, treatment 15 of 40 (37.5%), control 8 of 17 (47.1%), NNT 10, no improvement ≥2 points, day 15.
|
|
risk of no improvement, 15.0% lower, RR 0.85, p = 0.25, treatment 33 of 41 (80.5%), control 18 of 19 (94.7%), NNT 7.0, no improvement ≥2 points, day 5.
|
|
hospitalization time, 15.9% lower, relative time 0.84, p = 0.07, treatment median 11.1 IQR 5.0 n=41, control median 13.2 IQR 6.5 n=19.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jain et al., 30 Sep 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 50.9, 19 authors, study period 22 July, 2020 - 29 December, 2020, average treatment delay 5.15 days, trial NCT04456153 (history).
Contact: mamta.jain@utsouthwestern.edu, hesham.sadek@utsouthwestern.edu, john.schoggins@utsouthwestern.edu.
Atovaquone for treatment of COVID-19: A prospective randomized, double-blind, placebo-controlled clinical trial
Frontiers in Pharmacology, doi:10.3389/fphar.2022.1020123
Background: An in silico screen was performed to identify FDA approved drugs that inhibit SARS-CoV-2 main protease (M pro ), followed by in vitro viral replication assays, and in vivo pharmacokinetic studies in mice. These studies identified atovaquone as a promising candidate for inhibiting viral replication. Methods: A 2-center, randomized, double-blind, placebo-controlled trial was performed among patients hospitalized with COVID-19 infection. Enrolled patients were randomized 2:1 to atovaquone 1500 mg BID versus matched placebo. Patients received standard of care treatment including remdesivir, dexamethasone, or convalescent plasma as deemed necessary by the treating team. Saliva was collected at baseline and twice per day for up to 10 days for RNA extraction for SARS-CoV-2 viral load measurement by quantitative reverse-transcriptase PCR. The primary outcome was the between group difference in log-transformed viral load (copies/mL) using a generalized linear mixed-effect models of repeated measures from all samples. Results: Of the 61 patients enrolled; 41 received atovaquone and 19 received placebo. Overall, the population was predominately male (63%) and Hispanic (70%), with a mean age of 51 years, enrolled a mean of 5 days from symptom onset. The log 10 viral load was 5.25 copies/mL vs. 4.79 copies/mL at baseline in
Ethics statement The studies involving human participants were reviewed and approved by UT Southwestern Medical Center Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions MKJ, JD, DM, JS, and HS were involved in design, analysis, and writing the manuscript. CA and CX performed statistical analysis. All other authors contributed to data collection and manuscript review.
Conflict of interest MKJ has received research funding from Gilead Sciences and Regeneron and was on Advisory Board for Gilead Sciences. SM received research funding from Regeneron. JADL has received consulting income from Regeneron and Eli Lilly unrelated to COVID-19. JWS serves as a consultant for the Federal Trade Commission on matters related to COVID-19 treatments. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, Wang, Boys, Eitson, Ohlson et al., Identification of atovaquone as and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2 chemRxiv
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N. Engl. J. Med, doi:10.1056/NEJMoa2116044
Calderon, Penzak, Pau, Kumar, Mcmanus et al., Efavirenz but not atazanavir/ritonavir significantly reduces atovaquone concentrations in HIV-infected subjects, Clin. Infect. Dis, doi:10.1093/cid/ciw028
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat. Microbiol, doi:10.1038/s41564-020-00835-2
Dal-Re, Becker, Bottieau, Holm, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect. Dis, doi:10.1016/s1473-3099(22)00119-0
Davey, Jr, Fernandez-Cruz, Markowitz, Pett et al., Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): A double-blind, randomised, placebo-controlled trial, Lancet. Respir. Med, doi:10.1016/S2213-2600(19)30253-X
Group, Lundgren, Grund, Barkauskas, Holland et al., Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels : A randomized controlled trial, Ann. Intern. Med, doi:10.7326/M21-3507
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Malik, Sircar, Bhat, Sharun, Dhama et al., Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments, Vet. Q, doi:10.1080/01652176.2020.1727993
Mengist, Dilnessa, Structural basis of potential inhibitors targeting SARS-CoV-2 main protease, Front. Chem, doi:10.3389/fchem.2021.622898
Mohammad, Shamsi, Anwar, Umair, Hussain et al., Identification of high-affinity inhibitors of SARS-CoV-2 main protease: Towards the development of effective COVID-19 therapy, Virus Res, doi:10.1016/j.virusres.2020.198102
Owen Dafydd, Allerton Charlotte, Anderson Annaliesa, Aschenbrenner, Avery et al., An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19, Science, doi:10.1126/science.abl4784
Robin, Le, Melica, Massias, Redjoul et al., Plasma concentrations of atovaquone given to immunocompromised patients to prevent Pneumocystis jirovecii, J. Antimicrob. Chemother, doi:10.1093/jac/dkx198
Rosenke, Hansen, Schwarz, Feldmann, Haddock et al., Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model, Nat. Commun, doi:10.1038/s41467-021-22580-8
Wu, Zhao, Yu, Chen, Wang et al., A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N. Engl. J. Med, doi:10.1056/NEJMc2016359
Yoshino, Yasuo, Sekijima, Identification of key interactions between SARS-CoV-2 main protease and inhibitor drug candidates, Sci. Rep, doi:10.1038/s41598-020-69337-9
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
DOI record:
{
"DOI": "10.3389/fphar.2022.1020123",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2022.1020123",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> An <jats:italic>in silico</jats:italic> screen was performed to identify FDA approved drugs that inhibit SARS-CoV-2 main protease (M<jats:sup>pro</jats:sup>), followed by <jats:italic>in vitro</jats:italic> viral replication assays, and <jats:italic>in vivo</jats:italic> pharmacokinetic studies in mice. These studies identified atovaquone as a promising candidate for inhibiting viral replication.</jats:p><jats:p><jats:bold>Methods:</jats:bold> A 2-center, randomized, double-blind, placebo-controlled trial was performed among patients hospitalized with COVID-19 infection. Enrolled patients were randomized 2:1 to atovaquone 1500 mg BID <jats:italic>versus</jats:italic> matched placebo. Patients received standard of care treatment including remdesivir, dexamethasone, or convalescent plasma as deemed necessary by the treating team. Saliva was collected at baseline and twice per day for up to 10 days for RNA extraction for SARS-CoV-2 viral load measurement by quantitative reverse-transcriptase PCR. The primary outcome was the between group difference in log-transformed viral load (copies/mL) using a generalized linear mixed-effect models of repeated measures from all samples.</jats:p><jats:p><jats:bold>Results:</jats:bold> Of the 61 patients enrolled; 41 received atovaquone and 19 received placebo. Overall, the population was predominately male (63%) and Hispanic (70%), with a mean age of 51 years, enrolled a mean of 5 days from symptom onset. The log<jats:sub>10</jats:sub> viral load was 5.25 copies/mL <jats:italic>vs</jats:italic>. 4.79 copies/mL at baseline in the atovaquone <jats:italic>vs</jats:italic>. placebo group. Change in viral load did not differ over time between the atovaquone plus standard of care arm <jats:italic>versus</jats:italic> the placebo plus standard of care arm. Pharmacokinetic (PK) studies of atovaquone plasma concentration demonstrated a wide variation in atovaquone levels, with an inverse correlation between BMI and atovaquone levels, (Rho −0.45, <jats:italic>p</jats:italic> = 0.02). In post hoc analysis, an inverse correlation was observed between atovaquone levels and viral load (Rho −0.54, <jats:italic>p</jats:italic> = 0.005).</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> In this prospective, randomized, placebo-controlled trial, atovaquone did not demonstrate evidence of enhanced SARS-CoV-2 viral clearance compared with placebo. However, based on the observed inverse correlation between atovaquone levels and viral load, additional PK-guided studies may be warranted to examine the antiviral effect of atovaquone in COVID-19 patients.</jats:p>",
"alternative-id": [
"10.3389/fphar.2022.1020123"
],
"author": [
{
"affiliation": [],
"family": "Jain",
"given": "Mamta K.",
"sequence": "first"
},
{
"affiliation": [],
"family": "De Lemos",
"given": "James A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGuire",
"given": "Darren K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ayers",
"given": "Colby.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eitson",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanchez",
"given": "Claudia L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamel",
"given": "Dena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meisner",
"given": "Jessica A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Emilia V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hegde",
"given": "Anita A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mocherla",
"given": "Satish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strebe",
"given": "Joslyn K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xilong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Noelle S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xing",
"given": "Chao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Mahmoud S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sadek",
"given": "Hesham A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schoggins",
"given": "John W.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T05:10:47Z",
"timestamp": 1664514647000
},
"deposited": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T05:10:49Z",
"timestamp": 1664514649000
},
"funder": [
{
"DOI": "10.13039/100007914",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100007914",
"id-type": "DOI"
}
],
"name": "University of Texas Southwestern Medical Center"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T17:36:36Z",
"timestamp": 1740159396605,
"version": "3.37.3"
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2022,
9,
30
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T00:00:00Z",
"timestamp": 1664496000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.1020123/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
9,
30
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
30
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "B1",
"unstructured": "Identification of atovaquone as and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2 chemRxiv\n AhmedM.\n WangP.\n BoysI. N.\n EitsonJ. L.\n OhlsonM. B.\n FanW.\n 2021"
},
{
"DOI": "10.1093/cid/ciw028",
"article-title": "Efavirenz but not atazanavir/ritonavir significantly reduces atovaquone concentrations in HIV-infected subjects",
"author": "Calderon",
"doi-asserted-by": "publisher",
"first-page": "1036",
"journal-title": "Clin. Infect. Dis.",
"key": "B2",
"volume": "62",
"year": "2016"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"article-title": "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets",
"author": "Cox",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Nat. Microbiol.",
"key": "B3",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/s1473-3099(22)00119-0",
"article-title": "Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach",
"author": "Dal-Re",
"doi-asserted-by": "publisher",
"first-page": "e231",
"journal-title": "Lancet Infect. Dis.",
"key": "B4",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(19)30253-X",
"article-title": "Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): A double-blind, randomised, placebo-controlled trial",
"author": "Davey",
"doi-asserted-by": "publisher",
"first-page": "951",
"journal-title": "Lancet. Respir. Med.",
"key": "B5",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.7326/M21-3507",
"article-title": "Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels : A randomized controlled trial",
"author": "Group",
"doi-asserted-by": "publisher",
"first-page": "234",
"journal-title": "Ann. Intern. Med.",
"key": "B6",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "B7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "B8",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N. Engl. J. Med.",
"key": "B9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1080/01652176.2020.1727993",
"article-title": "Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments",
"author": "Malik",
"doi-asserted-by": "publisher",
"first-page": "68",
"journal-title": "Vet. Q.",
"key": "B10",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.3389/fchem.2021.622898",
"article-title": "Structural basis of potential inhibitors targeting SARS-CoV-2 main protease",
"author": "Mengist",
"doi-asserted-by": "publisher",
"first-page": "622898",
"journal-title": "Front. Chem.",
"key": "B11",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2020.198102",
"article-title": "Identification of high-affinity inhibitors of SARS-CoV-2 main protease: Towards the development of effective COVID-19 therapy",
"author": "Mohammad",
"doi-asserted-by": "publisher",
"first-page": "198102",
"journal-title": "Virus Res.",
"key": "B12",
"volume": "288",
"year": "2020"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19",
"author": "Owen Dafydd",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Science",
"key": "B13",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkx198",
"article-title": "Plasma concentrations of atovaquone given to immunocompromised patients to prevent Pneumocystis jirovecii",
"author": "Robin",
"doi-asserted-by": "publisher",
"first-page": "2602",
"journal-title": "J. Antimicrob. Chemother.",
"key": "B14",
"volume": "72",
"year": "2017"
},
{
"DOI": "10.1038/s41467-021-22580-8",
"article-title": "Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model",
"author": "Rosenke",
"doi-asserted-by": "publisher",
"first-page": "2295",
"journal-title": "Nat. Commun.",
"key": "B15",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"article-title": "A new coronavirus associated with human respiratory disease in China",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "265",
"journal-title": "Nature",
"key": "B16",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2016359",
"article-title": "Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2",
"author": "Wyllie",
"doi-asserted-by": "publisher",
"first-page": "1283",
"journal-title": "N. Engl. J. Med.",
"key": "B17",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-69337-9",
"article-title": "Identification of key interactions between SARS-CoV-2 main protease and inhibitor drug candidates",
"author": "Yoshino",
"doi-asserted-by": "publisher",
"first-page": "12493",
"journal-title": "Sci. Rep.",
"key": "B18",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "270",
"journal-title": "Nature",
"key": "B19",
"volume": "579",
"year": "2020"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2022.05.24.22275411",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.1020123/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Atovaquone for treatment of COVID-19: A prospective randomized, double-blind, placebo-controlled clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "13"
}