Impact of variable titer COVID-19 convalescent plasma and recipient SARS-CoV2-specific humoral immunity on survival in hospitalized patients
et al., PLOS ONE, doi:10.1371/journal.pone.0309449, Oct 2024
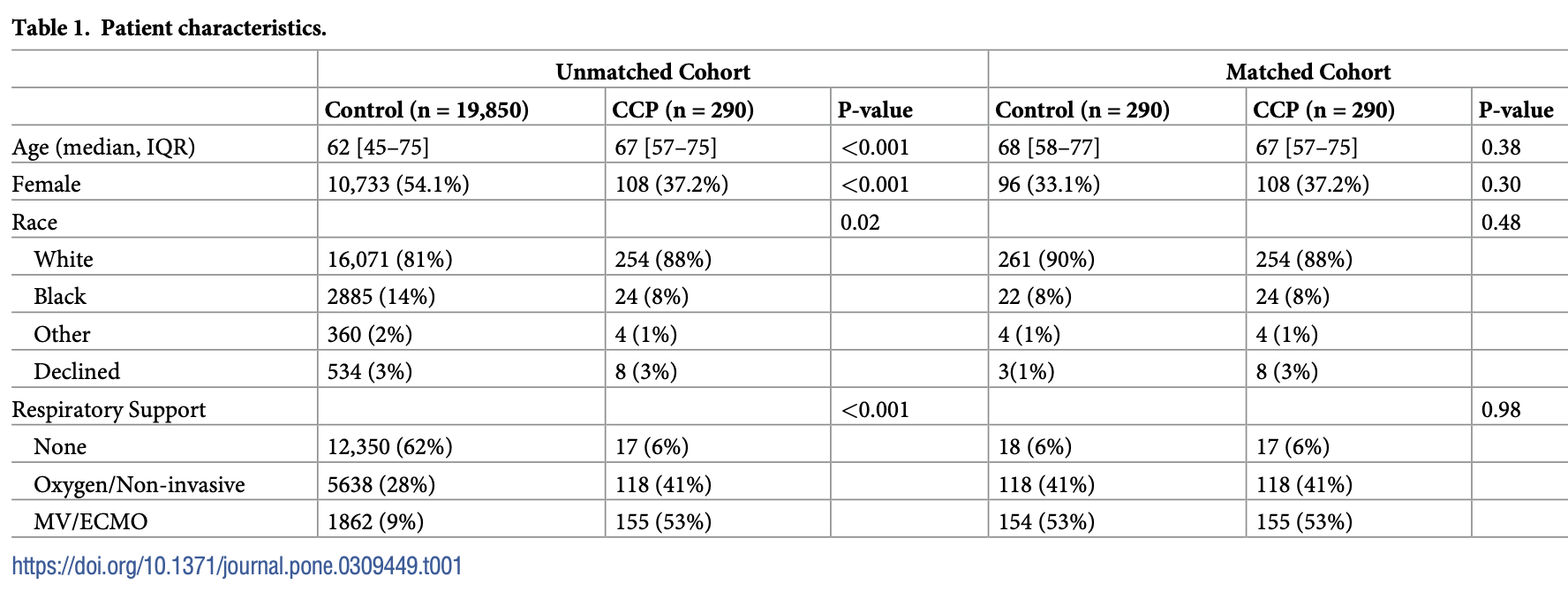
Retrospective propensity-matched analysis of 290 hospitalized COVID-19 patients who received convalescent plasma (CCP) compared to 290 controls, showing no significant difference in 30-day mortality, ECMO/mechanical ventilation, or hospital length of stay.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 25.9% higher, RR 1.26, p = 0.14, treatment 73 of 290 (25.2%), control 58 of 290 (20.0%), propensity score matching, day 30.
|
|
risk of mechanical ventilation, 0.6% higher, RR 1.01, p = 1.00, treatment 155 of 290 (53.4%), control 154 of 290 (53.1%), MV/ECMO, propensity score matching, day 30.
|
|
oxygen, no change, RR 1.00, p = 1.00, treatment 118 of 290 (40.7%), control 118 of 290 (40.7%), propensity score matching, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Iasella et al., 24 Oct 2024, retrospective, USA, peer-reviewed, 30 authors, study period March 2020 - June 2021.
Contact: askirb@pitt.edu, mcdyerjf@upmc.edu.
Impact of variable titer COVID-19 convalescent plasma and recipient SARS-CoV2-specific humoral immunity on survival in hospitalized patients
PLOS ONE, doi:10.1371/journal.pone.0309449
COVID-19 convalescent plasma (CCP) was one of the first therapies to receive emergency use authorization for management of COVID-19. We assessed the effectiveness of CCP in a propensity-matched analysis, and whether the presence of antibodies in the recipient at the time of treatment or the titer of antibodies in the administered CCP influenced clinical effectiveness. In an inpatient population within a single large health system, a total of 290 CCP patients were matched to 290 controls. While CCP increased titers of anti-SARS-CoV-2 RBD IgG titers post-CCP (p = <0.0001), no differences in 30-day survival were observed between CCP patients and controls in univariate and multivariate analyses. Survival at 30 days was numerically lower in recipients who were seronegative prior to CCP administration, compared to those with low titer and high titer anti-SARS-CoV-2 RBD IgG, respectively, but did not reach statistical significance (56% vs 82% vs 75%, p = 0.16). Patients who received 2 units of high-titer CCP had numerically better survival versus those who received fewer high-titer units, but this was not statistically significant (p = 0.08). CCP did not improve 30-day survival compared to propensity matched controls. Together these data support that CCP therapy provides limited benefit to hospitalized patients with SARS-CoV-2 infection.
Supporting information
References
Abani, Abbas, Abbas, Abbas, Abbasi et al., Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736%2821%2900897-7
Abani, Abbas, Abbas, Abbas, Abbasi et al., Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736%2821%2900897-7
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ, doi:10.1136/bmj.m3939
Alemany, Millat-Martinez, Corbacho-Monne, Malchair, Ouchi et al., Hightitre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600%2821%2900545-2
Avendaño-Sola, ´nez, Muñez-Rubio, Ruiz-Antora ´n, Molina et al., A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest, doi:10.1172/JCI152740
Bar, Shaw, Choi, Aqui, Fesnak et al., A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia, J Clin Invest, doi:10.1172/JCI155114
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest, doi:10.1172/JCI138003
Denkinger, Janssen, Scha ¨kel, Gall, Leo et al., Anti-SARS-CoV-2 antibodycontaining plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial, Nat Cancer, doi:10.1038/s43018-022-00503-w
Devos, Van Thillo, Compernolle, Najdovski, Romano et al., Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma, Eur Respir J, doi:10.1183/13993003.01724-2021
Ginde, Paredes, Murray, Engen, Grandits et al., Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600%2822%2900215-6
Hamilton, Lee, Arnold, Lilford, Hemming, Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial, Int J Infect Dis, doi:10.1016/j.ijid.2021.06.034
Hueso, Pouderoux, Pe ´re, Beaumont, Raillon et al., Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19, Blood, doi:10.1182/blood.2020008423
Iasella, Hannan, Saul, Koshy, Burke et al., Funding acquisition: John F. McDyer
Ko ¨rper, Weiss, Zickler, Wiesmann, Zacharowski et al., Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19, J Clin Invest, doi:10.1172/JCI152264
Korley, Durkalski-Mauldin, Yeatts, Schulman, Davenport et al., Early Convalescent Plasma for High-Risk Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2103784
Korley, Durkalski-Mauldin, Yeatts, Schulman, Davenport et al., Early Convalescent Plasma for High-Risk Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2103784
Levine, Fukuta, Huaman, Ou, Meisenberg et al., Coronavirus Disease 2019 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-Analysis of Individual Participant Data From 5 Randomized Trials, Clin Infect Dis, doi:10.1093/cid/ciad088
Li, Scha, Kulkarni, Liu, Martinez et al., High Potency of a Bivalent Human V H Domain in SARS-CoV-2 Animal Models In Brief High Potency of a Bivalent Human V H Domain in SARS-CoV-2 Animal Models, Cell, doi:10.1016/j.cell.2020.09.007
Li, Zhang, Hu, Tong, Zheng et al., Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial, JAMA-J Am Med Assoc, doi:10.1001/jama.2020.10044
Libster, ´rez Marc, Wappner, Coviello, Bianchi et al., Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Merin, Levee, Merlo, Spector, Coleman et al., The feasibility of multiple units of convalescent plasma in mechanically ventilated patients with COVID-19: A pilot study, Transfus Apher Sci, doi:10.1016/j.transci.2022.103423
Premkumar, Segovia-Chumbez, Jadi, Martinez, Raut et al., The receptorbinding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients, Sci Immunol, doi:10.1126/sciimmunol.abc8413
Razonable, Pawlowski, Horo, Arndt, Arndt et al., Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, eClinicalMedicine, doi:10.1016/j.eclinm.2021.101102
Senefeld, Franchini, Mengoli, Cruciani, Zani et al., COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.50647
Senefeld, Gorman, Johnson, Moir, Klassen et al., Mortality Rates Among Hospitalized Patients With COVID-19 Treated With Convalescent Plasma: A Systematic Review and Meta-Analysis, Mayo Clin Proc Innov Qual Outcomes, doi:10.1016/J.MAYOCPIQO.2023.09.001
Starr, Czudnochowski, Liu, Zatta, Park et al., SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape, Nat, doi:10.1038/s41586-021-03807-6
Sullivan, Gebo, Shoham, Bloch, Lau et al., Early Outpatient Treatment for Covid-19 with Convalescent Plasma, N Engl J Med, doi:10.1056/NEJMoa2119657
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
DOI record:
{
"DOI": "10.1371/journal.pone.0309449",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0309449",
"abstract": "<jats:p>COVID-19 convalescent plasma (CCP) was one of the first therapies to receive emergency use authorization for management of COVID-19. We assessed the effectiveness of CCP in a propensity-matched analysis, and whether the presence of antibodies in the recipient at the time of treatment or the titer of antibodies in the administered CCP influenced clinical effectiveness. In an inpatient population within a single large health system, a total of 290 CCP patients were matched to 290 controls. While CCP increased titers of anti-SARS-CoV-2 RBD IgG titers post-CCP (p = <0.0001), no differences in 30-day survival were observed between CCP patients and controls in univariate and multivariate analyses. Survival at 30 days was numerically lower in recipients who were seronegative prior to CCP administration, compared to those with low titer and high titer anti-SARS-CoV-2 RBD IgG, respectively, but did not reach statistical significance (56% vs 82% vs 75%, p = 0.16). Patients who received 2 units of high-titer CCP had numerically better survival versus those who received fewer high-titer units, but this was not statistically significant (p = 0.08). CCP did not improve 30-day survival compared to propensity matched controls. Together these data support that CCP therapy provides limited benefit to hospitalized patients with SARS-CoV-2 infection.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2668-3570",
"affiliation": [],
"authenticated-orcid": true,
"family": "Iasella",
"given": "Carlo J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hannan",
"given": "Stefanie J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyons",
"given": "Emily J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lieber",
"given": "Sophia C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Das",
"given": "Antu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dimitrov",
"given": "Dimiter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Wei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6712-5102",
"affiliation": [],
"authenticated-orcid": true,
"family": "Saul",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Popescu",
"given": "Iulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koshy",
"given": "Ritchie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burke",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lape",
"given": "Braidon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Mark J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xiaoping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sembrat",
"given": "John C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devonshire",
"given": "Kaitlyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kitsios",
"given": "Georgios D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6785-1318",
"affiliation": [],
"authenticated-orcid": true,
"family": "Konstantinidis",
"given": "Ioannis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Snyder",
"given": "Mark E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Bill B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Merlo",
"given": "Christian A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hager",
"given": "David N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiss",
"given": "Joseph E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yazer",
"given": "Mark H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wells",
"given": "Alan H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morris",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McVerry",
"given": "Bryan J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McMahon",
"given": "Deborah K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Triulzi",
"given": "Darrell J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McDyer",
"given": "John F.",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2024,
10,
24
]
],
"date-time": "2024-10-24T17:39:46Z",
"timestamp": 1729791586000
},
"deposited": {
"date-parts": [
[
2024,
10,
24
]
],
"date-time": "2024-10-24T17:39:57Z",
"timestamp": 1729791597000
},
"editor": [
{
"affiliation": [],
"family": "Jaworski",
"given": "Juan P.",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100000945",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000945",
"id-type": "DOI"
}
],
"name": "Pittsburgh Foundation"
},
{
"name": "David Scaife Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
10,
25
]
],
"date-time": "2024-10-25T04:23:26Z",
"timestamp": 1729830206292,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2024,
10,
24
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2024,
10,
24
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
24
]
],
"date-time": "2024-10-24T00:00:00Z",
"timestamp": 1729728000000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0309449",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0309449",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2024,
10,
24
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
24
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1172/JCI138003",
"article-title": "The convalescent sera option for containing COVID-19",
"author": "A Casadevall",
"doi-asserted-by": "crossref",
"first-page": "1545",
"journal-title": "J Clin Invest",
"key": "pone.0309449.ref001",
"volume": "130",
"year": "2020"
},
{
"key": "pone.0309449.ref002",
"unstructured": "EUA 26382 COVID-19 Convalescent Plasma. In: US Food and Drug Administration [Internet]. Aug 2020 [cited 22 Mar 2023]. Available: https://www.fda.gov/media/141480/download."
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial.",
"author": "O Abani",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "Lancet",
"key": "pone.0309449.ref003",
"volume": "397",
"year": "2021"
},
{
"article-title": "Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial).",
"author": "A Agarwal",
"first-page": "371",
"journal-title": "BMJ",
"key": "pone.0309449.ref004",
"year": "2020"
},
{
"article-title": "A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia",
"author": "C Avendaño-Solá",
"first-page": "131",
"journal-title": "J Clin Invest",
"key": "pone.0309449.ref005",
"year": "2021"
},
{
"article-title": "Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial.",
"author": "L Li",
"journal-title": "JAMA—J Am Med Assoc.",
"key": "pone.0309449.ref006",
"volume": "324",
"year": "2020"
},
{
"article-title": "Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults",
"author": "R Libster",
"first-page": "384",
"journal-title": "N Engl J Med",
"key": "pone.0309449.ref007",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2119657",
"article-title": "Early Outpatient Treatment for Covid-19 with Convalescent Plasma",
"author": "DJ Sullivan",
"doi-asserted-by": "crossref",
"first-page": "1700",
"journal-title": "N Engl J Med",
"key": "pone.0309449.ref008",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.50647",
"article-title": "COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis",
"author": "JW Senefeld",
"doi-asserted-by": "crossref",
"first-page": "e2250647",
"journal-title": "JAMA Netw Open",
"key": "pone.0309449.ref009",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early Convalescent Plasma for High-Risk Outpatients with Covid-19",
"author": "FK Korley",
"doi-asserted-by": "crossref",
"first-page": "1951",
"journal-title": "N Engl J Med",
"key": "pone.0309449.ref010",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00545-2",
"article-title": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial",
"author": "A Alemany",
"doi-asserted-by": "crossref",
"first-page": "278",
"journal-title": "Lancet Respir Med",
"key": "pone.0309449.ref011",
"volume": "10",
"year": "2022"
},
{
"article-title": "National Center for Advancing Translational Sciences, National Institutes of Health [Internet].",
"author": "National COVID Cohort Collaborative",
"key": "pone.0309449.ref012",
"year": "2022"
},
{
"article-title": "High Potency of a Bivalent Human V H Domain in SARS-CoV-2 Animal Models In Brief High Potency of a Bivalent Human V H Domain in SARS-CoV-2",
"author": "W Li",
"first-page": "429",
"journal-title": "Animal Models. Cell",
"key": "pone.0309449.ref013",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abc8413",
"article-title": "The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients.",
"author": "L Premkumar",
"doi-asserted-by": "crossref",
"first-page": "8413",
"journal-title": "Sci Immunol",
"key": "pone.0309449.ref014",
"volume": "5",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape.",
"author": "TN Starr",
"first-page": "97",
"journal-title": "Nat 2021 5977874",
"key": "pone.0309449.ref015",
"volume": "597",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocpiqo.2023.09.001",
"article-title": "Mortality Rates Among Hospitalized Patients With COVID-19 Treated With Convalescent Plasma: A Systematic Review and Meta-Analysis",
"author": "JW Senefeld",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "Mayo Clin Proc Innov Qual Outcomes",
"key": "pone.0309449.ref016",
"volume": "7",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early Convalescent Plasma for High-Risk Outpatients with Covid-19",
"author": "FK Korley",
"doi-asserted-by": "crossref",
"first-page": "1951",
"journal-title": "N Engl J Med",
"key": "pone.0309449.ref017",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciad088",
"article-title": "Coronavirus Disease 2019 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-Analysis of Individual Participant Data From 5 Randomized Trials",
"author": "AC Levine",
"doi-asserted-by": "crossref",
"first-page": "2077",
"journal-title": "Clin Infect Dis",
"key": "pone.0309449.ref018",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19",
"author": "DM Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "pone.0309449.ref019",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19.",
"author": "RR Razonable",
"doi-asserted-by": "crossref",
"first-page": "101102",
"journal-title": "eClinicalMedicine.",
"key": "pone.0309449.ref020",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00215-6",
"article-title": "Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial",
"author": "AA Ginde",
"doi-asserted-by": "crossref",
"first-page": "972",
"journal-title": "Lancet Respir Med",
"key": "pone.0309449.ref021",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial.",
"author": "O Abani",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "Lancet (London, England).",
"key": "pone.0309449.ref022",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"article-title": "Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial",
"author": "FW Hamilton",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Int J Infect Dis",
"key": "pone.0309449.ref023",
"volume": "109",
"year": "2021"
},
{
"article-title": "Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial.",
"author": "CM Denkinger",
"journal-title": "Nat Cancer.",
"key": "pone.0309449.ref024",
"volume": "4",
"year": "2022"
},
{
"DOI": "10.1182/blood.2020008423",
"article-title": "Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19",
"author": "T Hueso",
"doi-asserted-by": "crossref",
"first-page": "2290",
"journal-title": "Blood",
"key": "pone.0309449.ref025",
"volume": "136",
"year": "2020"
},
{
"article-title": "A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia",
"author": "KJ Bar",
"journal-title": "J Clin Invest",
"key": "pone.0309449.ref026",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1183/13993003.01724-2021",
"article-title": "Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma",
"author": "T Devos",
"doi-asserted-by": "crossref",
"journal-title": "Eur Respir J",
"key": "pone.0309449.ref027",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19",
"author": "S Körper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "pone.0309449.ref028",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/j.transci.2022.103423",
"article-title": "The feasibility of multiple units of convalescent plasma in mechanically ventilated patients with COVID-19: A pilot study.",
"author": "NM Merin",
"doi-asserted-by": "crossref",
"first-page": "103423",
"journal-title": "Transfus Apher Sci",
"key": "pone.0309449.ref029",
"volume": "61",
"year": "2022"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0309449"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Impact of variable titer COVID-19 convalescent plasma and recipient SARS-CoV2-specific humoral immunity on survival in hospitalized patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "19"
}